Brain-derived neurotrophic factor (BDNF) induces polarized signaling of small GTPase (Rac1) protein at the onset of Schwann cell myelination through partitioning-defective 3 (Par3) protein
- PMID: 22128191
- PMCID: PMC3256919
- DOI: 10.1074/jbc.M111.312736
Brain-derived neurotrophic factor (BDNF) induces polarized signaling of small GTPase (Rac1) protein at the onset of Schwann cell myelination through partitioning-defective 3 (Par3) protein
Abstract
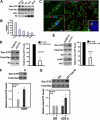
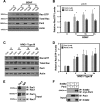
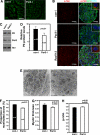
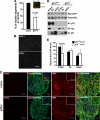
Brain-derived neurotrophic factor (BDNF) was shown to play a role in Schwann cell myelination by recruiting Par3 to the axon-glial interface, but the underlying mechanism has remained unclear. Here we report that Par3 regulates Rac1 activation by BDNF but not by NRG1-Type III in Schwann cells, although both ligands activate Rac1 in vivo. During development, active Rac1 signaling is localized to the axon-glial interface in Schwann cells by a Par3-dependent polarization mechanism. Knockdown of p75 and Par3 individually inhibits Rac1 activation, whereas constitutive activation of Rac1 disturbs the polarized activation of Rac1 in vivo. Polarized Rac1 activation is necessary for myelination as Par3 knockdown attenuates myelination in mouse sciatic nerves as well as in zebrafish. Specifically, Par3 knockdown in zebrafish disrupts proper alignment between the axon and Schwann cells without perturbing Schwann cell migration, suggesting that localized Rac1 activation at the axon-glial interface helps identify the initial wrapping sites. We therefore conclude that polarization of Rac1 activation is critical for myelination.
Figures

Similar articles
-
Elmo1 function, linked to Rac1 activity, regulates peripheral neuronal numbers and myelination in zebrafish.Cell Mol Life Sci. 2020 Jan;77(1):161-177. doi: 10.1007/s00018-019-03167-5. Epub 2019 Jun 3. Cell Mol Life Sci. 2020. PMID: 31161284 Free PMC article.
-
Rac1 and Rac3 have opposite functions in Schwann cells during developmental myelination.Neurosci Lett. 2021 May 14;753:135868. doi: 10.1016/j.neulet.2021.135868. Epub 2021 Apr 1. Neurosci Lett. 2021. PMID: 33812927
-
Dock1 functions in Schwann cells to regulate development, maintenance, and repair.J Cell Biol. 2025 May 5;224(5):e202311041. doi: 10.1083/jcb.202311041. Epub 2025 Mar 19. J Cell Biol. 2025. PMID: 40105697 Free PMC article.
-
New insights into signaling during myelination in zebrafish.Curr Top Dev Biol. 2011;97:1-19. doi: 10.1016/B978-0-12-385975-4.00007-3. Curr Top Dev Biol. 2011. PMID: 22074600 Free PMC article. Review.
-
Peripheral glia: Schwann cells in motion.Curr Biol. 2005 May 10;15(9):R332-4. doi: 10.1016/j.cub.2005.04.024. Curr Biol. 2005. PMID: 15886089 Review.
Cited by
-
CNTNAP2 is targeted to endosomes by the polarity protein PAR3.Eur J Neurosci. 2020 Feb;51(4):1074-1086. doi: 10.1111/ejn.14620. Epub 2019 Dec 2. Eur J Neurosci. 2020. PMID: 31730244 Free PMC article.
-
Necl-4/Cadm4 recruits Par-3 to the Schwann cell adaxonal membrane.Glia. 2019 May;67(5):884-895. doi: 10.1002/glia.23578. Epub 2018 Dec 26. Glia. 2019. PMID: 30585357 Free PMC article.
-
Cdc42 and RhoA reveal different spatio-temporal dynamics upon local stimulation with Semaphorin-3A.Front Cell Neurosci. 2015 Aug 26;9:333. doi: 10.3389/fncel.2015.00333. eCollection 2015. Front Cell Neurosci. 2015. PMID: 26379503 Free PMC article.
-
miR-219 regulates neural precursor differentiation by direct inhibition of apical par polarity proteins.Dev Cell. 2013 Nov 25;27(4):387-98. doi: 10.1016/j.devcel.2013.10.015. Epub 2013 Nov 14. Dev Cell. 2013. PMID: 24239515 Free PMC article.
-
Rho GTPases Signaling in Zebrafish Development and Disease.Cells. 2020 Dec 8;9(12):2634. doi: 10.3390/cells9122634. Cells. 2020. PMID: 33302361 Free PMC article. Review.
References
-
- Chan J. R., Jolicoeur C., Yamauchi J., Elliott J., Fawcett J. P., Ng B. K., Cayouette M. (2006) The polarity protein Par-3 directly interacts with p75NTR to regulate myelination. Science 314, 832–836 - PubMed
-
- Chen X., Macara I. G. (2005) Par-3 controls tight junction assembly through the Rac exchange factor Tiam1. Nat. Cell Biol. 7, 262–269 - PubMed
-
- Nishimura T., Kato K., Yamaguchi T., Fukata Y., Ohno S., Kaibuchi K. (2004) Role of the PAR-3-KIF3 complex in the establishment of neuronal polarity. Nat. Cell Biol. 6, 328–334 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

