Novel RGD-lipid conjugate-modified liposomes for enhancing siRNA delivery in human retinal pigment epithelial cells
- PMID: 22128247
- PMCID: PMC3225218
- DOI: 10.2147/IJN.S24447
Novel RGD-lipid conjugate-modified liposomes for enhancing siRNA delivery in human retinal pigment epithelial cells
Abstract
Background: Human retinal pigment epithelial cells are promising target sites for small interfering RNA (siRNA) that might be used for the prevention and/or treatment of choroidal neovascularization by inhibiting the expression of angiogenic factor; for example, by downregulating expression of the vascular endothelial growth factor gene.
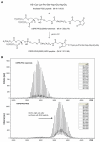
Methods: A novel functional lipid, DSPE-PEG-RGD, a Arg(R)-Gly(G)-Asp(D) motif peptide conjugated to 1, 2-distearoyl-sn-glycero-3-phosphoethanolamine- N-[maleimide (polyethylene glycol)-2000], was synthesized for the preparation of siRNA-loaded RGD-PEGylated liposomes to enhance uptake of encapsulated siRNA in retinal pigment epithelial cells. Various liposomes, with 1 mol% and 5 mol% PEGylated lipid or 1 mol% and 5 mol% RGD-PEGylated lipid, were fabricated.
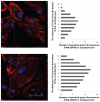
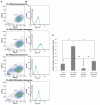
Results: Characterization of the liposomes, including siRNA entrapment efficiency, average particle size and ζ-potential, were determined to be as follows: >96%, 129.7 ± 51 to 230.7 ± 60.7 nm, and 17.3 ± 0.6 to 32 ± 1.3 mV, respectively. For the in vitro retinal pigment epithelial cell studies, the RGD-PEGylated liposomes had high delivery efficiency with siRNA delivery, about a four-fold increase compared with the PEGylated liposomes. Comparison of the various liposomes showed that the 1 mol% RGD-modified liposome had less cytotoxicity and higher siRNA delivery efficiency than the other liposomes. The antibody blocking assay confirmed that uptake of the 1 mol% RGD-PEGylated liposome was via integrin receptor- mediated endocytosis in retinal pigment epithelial cells.
Conclusion: The results of this study suggest that RGD-PEGylated liposomes might be useful for siRNA delivery into retinal pigment epithelial cells by integrin receptor-medicated endocytosis.
Keywords: Arg-Gly-Asp; RGD; liposome; retinal pigment epithelial cells; small interfering RNA.
Figures

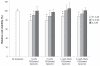

Similar articles
-
Efficient downregulation of VEGF in retinal pigment epithelial cells by integrin ligand-labeled liposome-mediated siRNA delivery.Int J Nanomedicine. 2013;8:2613-27. doi: 10.2147/IJN.S39622. Epub 2013 Jul 22. Int J Nanomedicine. 2013. PMID: 23901275 Free PMC article.
-
Dual-ligand modification of PEGylated liposomes shows better cell selectivity and efficient gene delivery.J Control Release. 2011 Jul 30;153(2):141-8. doi: 10.1016/j.jconrel.2011.03.012. Epub 2011 Apr 5. J Control Release. 2011. PMID: 21447361
-
Targeting effect of PEGylated liposomes modified with the Arg-Gly-Asp sequence on gastric cancer.Oncol Rep. 2015 Oct;34(4):1825-34. doi: 10.3892/or.2015.4142. Epub 2015 Jul 22. Oncol Rep. 2015. PMID: 26238930
-
Lipid-polymer hybrid nanoparticles as a new generation therapeutic delivery platform: a review.Eur J Pharm Biopharm. 2013 Nov;85(3 Pt A):427-43. doi: 10.1016/j.ejpb.2013.07.002. Epub 2013 Jul 17. Eur J Pharm Biopharm. 2013. PMID: 23872180 Review.
-
Effect of surface properties on liposomal siRNA delivery.Biomaterials. 2016 Feb;79:56-68. doi: 10.1016/j.biomaterials.2015.11.056. Epub 2015 Dec 2. Biomaterials. 2016. PMID: 26695117 Free PMC article. Review.
Cited by
-
Recent developments in microfluidic synthesis of artificial cell-like polymersomes and liposomes for functional bioreactors.Biomicrofluidics. 2021 Mar 30;15(2):021301. doi: 10.1063/5.0048441. eCollection 2021 Mar. Biomicrofluidics. 2021. PMID: 33833845 Free PMC article.
-
Influence of charge on FITC-BSA-loaded chondroitin sulfate-chitosan nanoparticles upon cell uptake in human Caco-2 cell monolayers.Int J Nanomedicine. 2012;7:4861-72. doi: 10.2147/IJN.S34770. Epub 2012 Aug 10. Int J Nanomedicine. 2012. PMID: 23028215 Free PMC article.
-
Promising Approach in the Treatment of Glaucoma Using Nanotechnology and Nanomedicine-Based Systems.Molecules. 2019 Oct 22;24(20):3805. doi: 10.3390/molecules24203805. Molecules. 2019. PMID: 31652593 Free PMC article. Review.
-
Lipid-based drug delivery systems in the treatment of wet age-related macular degeneration.Drug Deliv Transl Res. 2016 Dec;6(6):781-792. doi: 10.1007/s13346-016-0299-6. Drug Deliv Transl Res. 2016. PMID: 27194165 Review.
-
Improved Efficacy in a Fabry Disease Model Using a Systemic mRNA Liver Depot System as Compared to Enzyme Replacement Therapy.Mol Ther. 2019 Apr 10;27(4):878-889. doi: 10.1016/j.ymthe.2019.03.001. Epub 2019 Mar 6. Mol Ther. 2019. PMID: 30879951 Free PMC article.
References
-
- Zabner J. Cationic lipids used in gene transfer. Adv Drug Deliv Rev. 1997;27(1):17–28. - PubMed
-
- Liu F, Huang L. Development of non-viral vectors for systemic gene delivery. J Control Release. 2002;78(1–3):259–266. - PubMed
-
- Dass CR, Choong PF. Selective gene delivery for cancer therapy using cationic liposomes: in vivo proof of applicability. J Control Release. 2006;113(2):155–163. - PubMed
-
- Dykxhoorn DM, Novina CD, Sharp PA. Killing the messenger: short RNAs that silence gene expression. Nat Rev Mol Cell Biol. 2003;4(6):457–467. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

