Nanoparticles isolated from blood: a reflection of vesiculability of blood cells during the isolation process
- PMID: 22128248
- PMCID: PMC3225219
- DOI: 10.2147/IJN.S24537
Nanoparticles isolated from blood: a reflection of vesiculability of blood cells during the isolation process
Abstract
Background: Shedding of nanoparticles from the cell membrane is a common process in all cells. These nanoparticles are present in body fluids and can be harvested by isolation. To collect circulating nanoparticles from blood, a standard procedure consisting of repeated centrifugation and washing is applied to the blood samples. Nanoparticles can also be shed from blood cells during the isolation process, so it is unclear whether nanoparticles found in the isolated material are present in blood at sampling or if are they created from the blood cells during the isolation process. We addressed this question by determination of the morphology and identity of nanoparticles harvested from blood.
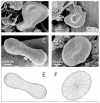
Methods: The isolates were visualized by scanning electron microscopy, analyzed by flow cytometry, and nanoparticle shapes were determined theoretically.
Results: The average size of nanoparticles was about 300 nm, and numerous residual blood cells were found in the isolates. The shapes of nanoparticles corresponded to the theoretical shapes obtained by minimization of the membrane free energy, indicating that these nanoparticles can be identified as vesicles. The concentration and size of nanoparticles in blood isolates was sensitive to the temperature during isolation. We demonstrated that at lower temperatures, the nanoparticle concentration was higher, while the nanoparticles were on average smaller.
Conclusion: These results indicate that a large pool of nanoparticles is produced after blood sampling. The shapes of deformed blood cells found in the isolates indicate how fragmentation of blood cells may take place. The results show that the contents of isolates reflect the properties of blood cells and their interaction with the surrounding solution (rather than representing only nanoparticles present in blood at sampling) which differ in different diseases and may therefore present a relevant clinical parameter.
Keywords: cell–cell communication; microparticles; microvesicles; nanoparticles; nanovesicles.
Figures





References
-
- Wolf P. The nature and significance of platelet products in human plasma. Br J Haematol. 1967;13(3):269–288. - PubMed
-
- Rumsby MG, Trotter J, Allan D, Michell RH. Recovery of membrane micro-vesicles from human erythrocytes stored for transfusion: a mechanism for the erythrocyte discocyte-to-spherocyte shape transformation. Biochem Soc Trans. 1977;5(1):126–128. - PubMed
-
- Greenwalt TJ. The how and why of exocytic vesicles. Transfusion. 2006;46(1):143–152. - PubMed
-
- Simak J, Gelderman MP. Cell membrane microparticles in blood and blood products: potentially pathogenic agents and diagnostic markers. Transfus Med Rev. 2006;20(1):1–26. - PubMed
-
- Allan D, Billah MM, Finean JB, Michell RH. Release of diacylglycerol-enriched vesicles from erythrocytes with increased intracellular Ca2+ Nature. 1976;261:58–60. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

