Interaction of curcumin nanoformulations with human plasma proteins and erythrocytes
- PMID: 22128249
- PMCID: PMC3225220
- DOI: 10.2147/IJN.S25534
Interaction of curcumin nanoformulations with human plasma proteins and erythrocytes
Abstract
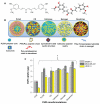
Background: Recent studies report curcumin nanoformulation(s) based on polylactic-co-glycolic acid (PLGA), β-cyclodextrin, cellulose, nanogel, and dendrimers to have anticancer potential. However, no comparative data are currently available for the interaction of curcumin nanoformulations with blood proteins and erythrocytes. The objective of this study was to examine the interaction of curcumin nanoformulations with cancer cells, serum proteins, and human red blood cells, and to assess their potential application for in vivo preclinical and clinical studies.
Methods: The cellular uptake of curcumin nanoformulations was assessed by measuring curcumin levels in cancer cells using ultraviolet-visible spectrophotometry. Protein interaction studies were conducted using particle size analysis, zeta potential, and Western blot techniques. Curcumin nanoformulations were incubated with human red blood cells to evaluate their acute toxicity and hemocompatibility.
Results: Cellular uptake of curcumin nanoformulations by cancer cells demonstrated preferential uptake versus free curcumin. Particle sizes and zeta potentials of curucumin nanoformulations were varied after human serum albumin adsorption. A remarkable capacity of the dendrimer curcumin nanoformulation to bind to plasma protein was observed, while the other formulations showed minimal binding capacity. Dendrimer curcumin nanoformulations also showed higher toxicity to red blood cells compared with the other curcumin nanoformulations.
Conclusion: PLGA and nanogel curcumin nanoformulations appear to be very compatible with erythrocytes and have low serum protein binding characteristics, which suggests that they may be suitable for application in the treatment of malignancy. These findings advance our understanding of the characteristics of curcumin nanoformulations, a necessary component in harnessing and implementing improved in vivo effects of curcumin.
Keywords: cellular uptake; chemotherapy; curcumin; hemocompatibility; nanoparticle; protein binding.
Figures





Similar articles
-
Scale up, optimization and stability analysis of Curcumin C3 complex-loaded nanoparticles for cancer therapy.J Nanobiotechnology. 2012 Aug 31;10:38. doi: 10.1186/1477-3155-10-38. J Nanobiotechnology. 2012. PMID: 22937885 Free PMC article.
-
Design of curcumin loaded cellulose nanoparticles for prostate cancer.Curr Drug Metab. 2012 Jan;13(1):120-8. doi: 10.2174/138920012798356952. Curr Drug Metab. 2012. PMID: 21892919 Free PMC article.
-
Preparation of DHAQ-loaded mPEG-PLGA-mPEG nanoparticles and evaluation of drug release behaviors in vitro/in vivo.J Mater Sci Mater Med. 2006 Jun;17(6):509-16. doi: 10.1007/s10856-006-8933-3. J Mater Sci Mater Med. 2006. PMID: 16691348
-
Curcumin as potential therapeutic natural product: a nanobiotechnological perspective.J Pharm Pharmacol. 2016 Dec;68(12):1481-1500. doi: 10.1111/jphp.12611. Epub 2016 Oct 17. J Pharm Pharmacol. 2016. PMID: 27747859 Review.
-
The effect of nanoparticle properties, detection method, delivery route and animal model on poly(lactic-co-glycolic) acid nanoparticles biodistribution in mice and rats.Drug Metab Rev. 2014 May;46(2):128-41. doi: 10.3109/03602532.2013.864664. Epub 2013 Dec 5. Drug Metab Rev. 2014. PMID: 24303927 Review.
Cited by
-
Advances in Nanocarrier Systems for Overcoming Formulation Challenges of Curcumin: Current Insights.Nanomaterials (Basel). 2024 Apr 12;14(8):672. doi: 10.3390/nano14080672. Nanomaterials (Basel). 2024. PMID: 38668166 Free PMC article. Review.
-
Curcumin nanomedicine: a road to cancer therapeutics.Curr Pharm Des. 2013;19(11):1994-2010. doi: 10.2174/138161213805289219. Curr Pharm Des. 2013. PMID: 23116309 Free PMC article.
-
Enhanced photocytotoxicity of curcumin delivered by solid lipid nanoparticles.Int J Nanomedicine. 2016 Dec 22;12:167-178. doi: 10.2147/IJN.S123107. eCollection 2017. Int J Nanomedicine. 2016. PMID: 28053531 Free PMC article.
-
Nanoformulations of curcumin: an emerging paradigm for improved remedial application.Oncotarget. 2017 Jul 11;8(39):66680-66698. doi: 10.18632/oncotarget.19164. eCollection 2017 Sep 12. Oncotarget. 2017. PMID: 29029547 Free PMC article. Review.
-
Bringing Curcumin to the Clinic in Cancer Prevention: a Review of Strategies to Enhance Bioavailability and Efficacy.AAPS J. 2017 Jan;19(1):54-81. doi: 10.1208/s12248-016-0003-2. Epub 2016 Oct 25. AAPS J. 2017. PMID: 27783266 Review.
References
-
- ClinicalTrialsgov [homepage on the Internet] Bethesda, MD: National Library of Medicine (US); [Accessed Nov 2, 2011]. Available from: http://clinicaltrials.gov/ct2/home.
-
- Bar-Sela G, Epelbaum R, Schaffer M. Curcumin as an anti-cancer agent: review of the gap between basic and clinical applications. Curr Med Chem. 2010;17(3):190–197. - PubMed
-
- Maheshwari RK, Singh AK, Gaddipati J, Srimal RC. Multiple biological activities of curcumin: a short review. Life Sci. 2006;78(18):2081–2087. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

