Effects of peripherally restricted κ opioid receptor agonists on pain-related stimulation and depression of behavior in rats
- PMID: 22128346
- PMCID: PMC3286312
- DOI: 10.1124/jpet.111.186783
Effects of peripherally restricted κ opioid receptor agonists on pain-related stimulation and depression of behavior in rats
Abstract
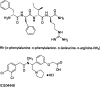
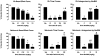
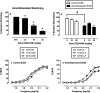
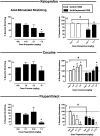
κ opioid receptor agonists that do not readily cross the blood-brain barrier are peripherally restricted and distribute poorly to the central nervous system after systemic administration. Peripherally restricted κ agonists have promise as candidate analgesics, because they may produce antinociception mediated by peripheral κ receptors more potently than they produce undesirable sedative and psychotomimetic effects mediated by central κ receptors. The present study used assays of pain-related stimulation and depression of behavior in rats to compare effects of 1) two peripherally restricted κ agonists [the tetrapeptide D-Phe-D-Phe-D-Ile-D-Arg-NH(2) (ffir) and the nonpeptidic compound ((R,S)-N-[2-(N-methyl-3,4-dichlorophenylacetamido)-2-(3-carboxyphenyl)-ethyl]pyrrolidine hydrochloride (ICI204448)], 2) a centrally penetrating κ agonist (salvinorin A), and 3) several reference drugs, including a nonsteroidal anti-inflammatory drug (NSAID; ketoprofen). Intraperitoneal injection of dilute lactic acid served as a noxious stimulus to stimulate a stretching response and depress intracranial self-stimulation (ICSS) maintained by the delivery of electrical brain stimulation to the medial forebrain bundle. Acid-stimulated stretching was blocked by ketoprofen, the peripherally restricted κ agonists, and salvinorin A. However, acid-induced depression of ICSS was blocked only by ketoprofen. The peripherally restricted κ agonists had little effect, and salvinorin A exacerbated acid-induced depression of ICSS. These results suggest that peripherally restricted κ agonists may be safer than centrally penetrating κ agonists but less efficacious than NSAIDS or μ opioid receptor agonists to block pain-related depression of behavior; however, the peripheral selectivity of ffir and ICI204448 is limited, and future studies with κ agonists capable of greater peripheral selectivity are warranted.
Figures


Similar articles
-
Pain-related depression of the mesolimbic dopamine system in rats: expression, blockade by analgesics, and role of endogenous κ-opioids.Neuropsychopharmacology. 2014 Feb;39(3):614-24. doi: 10.1038/npp.2013.236. Epub 2013 Sep 6. Neuropsychopharmacology. 2014. PMID: 24008352 Free PMC article.
-
Effects of kappa opioids in an assay of pain-depressed intracranial self-stimulation in rats.Psychopharmacology (Berl). 2010 Jun;210(2):149-59. doi: 10.1007/s00213-009-1770-6. Epub 2010 Jan 26. Psychopharmacology (Berl). 2010. PMID: 20101391 Free PMC article.
-
Effects of μ-opioid receptor agonists in assays of acute pain-stimulated and pain-depressed behavior in male rats: role of μ-agonist efficacy and noxious stimulus intensity.J Pharmacol Exp Ther. 2015 Feb;352(2):208-17. doi: 10.1124/jpet.114.219873. Epub 2014 Nov 18. J Pharmacol Exp Ther. 2015. PMID: 25406170 Free PMC article.
-
Peripheral kappa-opioid agonists for visceral pain.Br J Pharmacol. 2004 Apr;141(8):1331-4. doi: 10.1038/sj.bjp.0705763. Epub 2004 Mar 29. Br J Pharmacol. 2004. PMID: 15051626 Free PMC article. Review.
-
Salvinorin A analogs and other κ-opioid receptor compounds as treatments for cocaine abuse.Adv Pharmacol. 2014;69:481-511. doi: 10.1016/B978-0-12-420118-7.00012-3. Adv Pharmacol. 2014. PMID: 24484985 Free PMC article. Review.
Cited by
-
Pain-related depression of the mesolimbic dopamine system in rats: expression, blockade by analgesics, and role of endogenous κ-opioids.Neuropsychopharmacology. 2014 Feb;39(3):614-24. doi: 10.1038/npp.2013.236. Epub 2013 Sep 6. Neuropsychopharmacology. 2014. PMID: 24008352 Free PMC article.
-
Effects of the neuropeptide S receptor antagonist RTI-118 on abuse-related facilitation of intracranial self-stimulation produced by cocaine and methylenedioxypyrovalerone (MDPV) in rats.Eur J Pharmacol. 2014 Nov 15;743:98-105. doi: 10.1016/j.ejphar.2014.09.006. Epub 2014 Sep 16. Eur J Pharmacol. 2014. PMID: 25220242 Free PMC article.
-
The effect of chronic amphetamine treatment on cocaine-induced facilitation of intracranial self-stimulation in rats.Psychopharmacology (Berl). 2014 Jun;231(12):2461-70. doi: 10.1007/s00213-013-3405-1. Epub 2014 Jan 10. Psychopharmacology (Berl). 2014. PMID: 24408209 Free PMC article.
-
The application of conditioning paradigms in the measurement of pain.Eur J Pharmacol. 2013 Sep 15;716(1-3):158-68. doi: 10.1016/j.ejphar.2013.03.002. Epub 2013 Mar 13. Eur J Pharmacol. 2013. PMID: 23500202 Free PMC article. Review.
-
Lack of effect of the nociceptin opioid peptide agonist Ro 64-6198 on pain-depressed behavior and heroin choice in rats.Drug Alcohol Depend. 2022 Feb 1;231:109255. doi: 10.1016/j.drugalcdep.2021.109255. Epub 2021 Dec 30. Drug Alcohol Depend. 2022. PMID: 34998256 Free PMC article.
References
-
- Ansonoff MA, Zhang J, Czyzyk T, Rothman RB, Stewart J, Xu H, Zjwiony J, Siebert DJ, Yang F, Roth BL, et al. (2006) Antinociceptive and hypothermic effects of Salvinorin A are abolished in a novel strain of κ-opioid receptor-1 knockout mice. J Pharmacol Exp Ther 318:641–648 - PubMed
-
- Arendt-Nielsen L, Olesen AE, Staahl C, Menzaghi F, Kell S, Wong GY, Drewes AM. (2009) Analgesic efficacy of peripheral κ-opioid receptor agonist CR665 compared to oxycodone in a multi-modal, multi-tissue experimental human pain model: selective effect on visceral pain. Anesthesiology 111:616–624 - PubMed
-
- Baggott MJ, Erowid E, Erowid F, Galloway GP, Mendelson J. (2010) Use patterns and self-reported effects of Salvia divinorum: an internet-based survey. Drug Alcohol Depend 111:250–256 - PubMed
-
- Caram-Salas NL, Reyes-García G, Bartoszyk GD, Araiza-Saldaña CI, Ambriz-Tututi M, Rocha-González HI, Arreola-Espino R, Cruz SL, Granados-Soto V. (2007) Subcutaneous, intrathecal and periaqueductal grey administration of asimadoline and ICI-204448 reduces tactile allodynia in the rat. Eur J Pharmacol 573:75–83 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

