Activation of the prolyl-hydroxylase oxygen-sensing signal cascade leads to AMPK activation in cardiomyocytes
- PMID: 22128786
- PMCID: PMC3822975
- DOI: 10.1111/j.1582-4934.2011.01500.x
Activation of the prolyl-hydroxylase oxygen-sensing signal cascade leads to AMPK activation in cardiomyocytes
Abstract
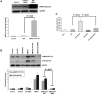
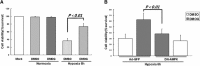
The proline hydroxylase domain-containing enzymes (PHD) act as cellular oxygen sensors and initiate a hypoxic signal cascade to induce a range of cellular responses to hypoxia especially in the aspect of energy and metabolic homeostasis regulation. AMP-activated protein kinase (AMPK) is recognized as a major energetic sensor and regulator of cardiac metabolism. However, the effect of PHD signal on AMPK has never been studied before. A PHD inhibitor (PHI), dimethyloxalylglycine and PHD2-specific RNA interference (RNAi) have been used to activate PHD signalling in neonatal rat cardiomyocytes. Both PHI and PHD2-RNAi activated AMPK pathway in cardiomyocytes effectively. In addition, the increased glucose uptake during normoxia and enhanced myocyte viability during hypoxia induced by PHI pretreatment were abrogated substantially upon AMPK inhibition with an adenoviral vector expressing a dominant negative mutant of AMPK-α1. Furthermore, chelation of intracellular Ca2+ by BAPTA, inhibition of calmodulin-dependent kinase kinase (CaMKK) with STO-609, or RNAi-mediated down-regulation of CaMKK α inhibited PHI-induced AMPK activation significantly. In contrast, down-regulation of LKB1 with adenoviruses expressing the dominant negative form did not affect PHI-induced AMPK activation. We establish for the first time that activation of PHD signal cascade can activate AMPK pathway mainly through a Ca(2+)/CaMKK-dependent mechanism in cardiomyocytes. Furthermore, activation of AMPK plays an essential role in hypoxic protective responses induced by PHI.
© 2012 The Authors Journal of Cellular and Molecular Medicine © 2012 Foundation for Cellular and Molecular Medicine/Blackwell Publishing Ltd.
Figures




References
-
- Bruick RK, McKnight SL. A conserved family of prolyl-4-hydroxylases that modify HIF. Science. 2001;294:1337–40. - PubMed
-
- Li SH, Shin DH, Chun YS, et al. A novel mode of action of YC-1 in HIF inhibition: stimulation of FIH-dependent p300 dissociation from HIF-1 alpha. Mol Cancer Ther. 2008;7:3729–38. - PubMed
-
- Ivan M, Kondo K, Yang HF, et al. HIF alpha targeted for VHL-mediated destruction by proline hydroxylation: implications for O-2 sensing. Science. 2001;292:464–8. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

