Regulation of mucosal IgA responses: lessons from primary immunodeficiencies
- PMID: 22129060
- PMCID: PMC3240841
- DOI: 10.1111/j.1749-6632.2011.06266.x
Regulation of mucosal IgA responses: lessons from primary immunodeficiencies
Abstract
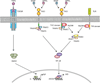
Adaptive co-evolution of mammals and bacteria has led to the establishment of complex commensal communities on mucosal surfaces. In spite of having available a wealth of immune-sensing and effector mechanisms capable of triggering inflammation in response to microbial intrusion, mucosal immune cells establish an intimate dialogue with microbes to generate a state of hyporesponsiveness against commensals and active readiness against pathogens. A key component of this homeostatic balance is IgA, a noninflammatory antibody isotype produced by mucosal B cells through class switching. This process involves activation of B cells by IgA-inducing signals originating from mucosal T cells, dendritic cells, and epithelial cells. Here, we review the mechanisms by which mucosal B cells undergo IgA diversification and production and discuss how the study of primary immunodeficiencies facilitates better understanding of mucosal IgA responses in humans.
© 2011 New York Academy of Sciences.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

References
-
- Sansonetti PJ. Host-bacteria homeostasis in the healthy and inflamed gut. Curr. Opin. Gastroenterol. 2008;24:435–439. - PubMed
-
- Macpherson AJ, Harris NL. Interactions between commensalintestinalbacteria andthe immunesystem. Nat. Rev. Immunol. 2004;4:478–485. - PubMed
-
- Hooper LV, Macpherson AJ. Immune adaptations that maintain homeostasis with the intestinal micro-biota. Nat. Rev. Immunol. 2010;10:159–169. - PubMed
-
- Abreu MT. Toll-like receptor signalling in the intestinal epithelium: how bacterial recognition shapes intestinal function. Nat. Rev. Immunol. 2010;10:131–144. - PubMed
-
- Fagarasan S, et al. Adaptive immune regulation in the gut: T cell-dependent and T cell-independent IgA synthesis. Annu. Rev. Immunol. 2010;28:243–273. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

