Lipopolysaccharide-enhanced transcellular transport of HIV-1 across the blood-brain barrier is mediated by luminal microvessel IL-6 and GM-CSF
- PMID: 22129063
- PMCID: PMC3260201
- DOI: 10.1186/1742-2094-8-167
Lipopolysaccharide-enhanced transcellular transport of HIV-1 across the blood-brain barrier is mediated by luminal microvessel IL-6 and GM-CSF
Abstract
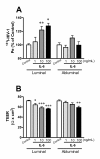
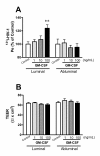
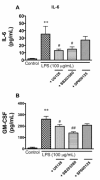
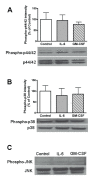
Elevated levels of cytokines/chemokines contribute to increased neuroinvasion of human immunodeficiency virus type 1 (HIV-1). Previous work showed that lipopolysaccharide (LPS), which is present in the plasma of patients with HIV-1, enhanced transcellular transport of HIV-1 across the blood-brain barrier (BBB) through the activation of p38 mitogen-activated protein kinase (MAPK) signaling in brain microvascular endothelial cells (BMECs). Here, we found that LPS (100 μg/mL, 4 hr) selectively increased interleukin (IL)-6 and granulocyte-macrophage colony-stimulating factor (GM-CSF) release from BMECs. The enhancement of HIV-1 transport induced by luminal LPS was neutralized by treatment with luminal, but not with abluminal, antibodies to IL-6 and GM-CSF without affecting paracellular permeability as measured by transendothelial electrical resistance (TEER). Luminal, but not abluminal, IL-6 or GM-CSF also increased HIV-1 transport. U0126 (MAPK kinase (MEK)1/2 inhibitor) and SB203580 (p38 MAPK inhibitor) decreased the LPS-enhanced release of IL-6 and GM-CSF. These results show that p44/42 and p38 MAPK signaling pathways mediate the LPS-enhanced release of IL-6 and GM-CSF. These cytokines, in turn, act at the luminal surface of the BMEC to enhance the transcellular transport of HIV-1 independently of actions on paracellular permeability.
Figures


References
-
- McArthur JC, Haughey N, Gartner S, Conant K, Pardo C, Nath A, Sacktor N. Human immunodeficiency virus-associated dementia: An evolving disease. J Neurovirol. 2003;9:205–221. - PubMed
-
- Banks WA, Akerstrom V, Kastin AJ. Adsorptive endocytosis mediates the passage of HIV-1 across the blood-brain barrier: evidence for a post-internalization coreceptor. Journal of Cell Science. 1998;111:533–540. - PubMed
-
- Banks WA, Freed EO, Wolf KM, Robinson SM, Franko M, Kumar VB. Transport of human immunodeficiency virus type 1 pseudoviruses across the blood-brain barrier: role of envelope proteins and adsorptive endocytosis. Journal of Virology. 2001;75:4681–4691. doi: 10.1128/JVI.75.10.4681-4691.2001. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

