Using surveillance data to estimate pandemic vaccine effectiveness against laboratory confirmed influenza A(H1N1)2009 infection: two case-control studies, Spain, season 2009-2010
- PMID: 22129083
- PMCID: PMC3262832
- DOI: 10.1186/1471-2458-11-899
Using surveillance data to estimate pandemic vaccine effectiveness against laboratory confirmed influenza A(H1N1)2009 infection: two case-control studies, Spain, season 2009-2010
Abstract
Background: Physicians of the Spanish Influenza Sentinel Surveillance System report and systematically swab patients attended to their practices for influenza-like illness (ILI). Within the surveillance system, some Spanish regions also participated in an observational study aiming at estimating influenza vaccine effectiveness (cycEVA study). During the season 2009-2010, we estimated pandemic influenza vaccine effectiveness using both the influenza surveillance data and the cycEVA study.
Methods: We conducted two case-control studies using the test-negative design, between weeks 48/2009 and 8/2010 of the pandemic season. The surveillance-based study included all swabbed patients in the sentinel surveillance system. The cycEVA study included swabbed patients from seven Spanish regions. Cases were laboratory-confirmed pandemic influenza A(H1N1)2009. Controls were ILI patients testing negative for any type of influenza. Variables collected in both studies included demographic data, vaccination status, laboratory results, chronic conditions, and pregnancy. Additionally, cycEVA questionnaire collected data on previous influenza vaccination, smoking, functional status, hospitalisations, visits to the general practitioners, and obesity. We used logistic regression to calculate adjusted odds ratios (OR), computing pandemic influenza vaccine effectiveness as (1-OR)*100.
Results: We included 331 cases and 995 controls in the surveillance-based study and 85 cases and 351 controls in the cycEVA study. We detected nine (2.7%) and two (2.4%) vaccine failures in the surveillance-based and cycEVA studies, respectively. Adjusting for variables collected in surveillance database and swabbing month, pandemic influenza vaccine effectiveness was 62% (95% confidence interval (CI): -5; 87). The cycEVA vaccine effectiveness was 64% (95%CI: -225; 96) when adjusting for common variables with the surveillance system and 75% (95%CI: -293; 98) adjusting for all variables collected.
Conclusion: Point estimates of the pandemic influenza vaccine effectiveness suggested a protective effect of the pandemic vaccine against laboratory-confirmed influenza A(H1N1)2009 in the season 2009-2010. Both studies were limited by the low vaccine coverage and the late start of the vaccination campaign. Routine influenza surveillance provides reliable estimates and could be used for influenza vaccine effectiveness studies in future seasons taken into account the surveillance system limitations.
Figures
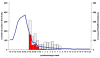
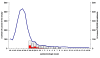
References
-
- Surveillance Group for New Influenza A(H1N1) Virus Investigation and Control in Spain. New influenza A(H1N1) virus infections in Spain, April-May 2009. Euro Surveill. 2009;14 http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=19209 pii = 19209. - PubMed
-
- Snacken R, Manuguerra JC, Taylor P. European Influenza Surveillance Scheme on the Internet. Methods Inf Med. 1998;37:266–70. - PubMed
-
- Larrauri A, De Mateo S. Characterisation of swabbing for virological analysis in the Spanish Influenza Sentinel Surveillance System during four influenza seasons in the period 2002-2006. Euro Surveill. 2007;12 http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=706 pii = 706. - PubMed
-
- European Centre for Disease Prevention and Control (ECDC) European Influenza Surveillance Network (EISN) http://ecdc.europa.eu/en/activities/surveillance/EISN/Pages/home.aspx
-
- Ministry of Health and Social Policy, Spain. Recommendations for influenza vaccination in the season 2009-10. http://www.msc.es/ciudadanos/enfLesiones/enfTransmisibles/gripe/gripe.ht... [In Spanish]
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

