Anti-tumor activity and toxicokinetics analysis of MGAH22, an anti-HER2 monoclonal antibody with enhanced Fcγ receptor binding properties
- PMID: 22129105
- PMCID: PMC3326565
- DOI: 10.1186/bcr3069
Anti-tumor activity and toxicokinetics analysis of MGAH22, an anti-HER2 monoclonal antibody with enhanced Fcγ receptor binding properties
Abstract
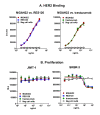
Introduction: Response to trastuzumab in metastatic breast cancer correlates with expression of the high binding variant (158V) of the activating Fcγ receptor IIIA (CD16A). We engineered MGAH22, a chimeric anti-HER2 monoclonal antibody with specificity and affinity similar to trastuzumab, with an Fc domain engineered for increased binding to both alleles of human CD16A.
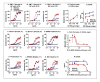
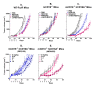
Methods: MGAH22 was compared to an identical anti-HER2 mAb except for a wild type Fc domain. Antibody-dependent cell cytotoxicity (ADCC) assays were performed with HER2-expressing cancer cells as targets and human PBMC or purified NK cells as effectors. Xenograft studies were conducted in mice with wild type murine FcγRs; in mice lacking murine CD16; or in mice lacking murine CD16 but transgenic for human CD16A-158F, the low-binding variant. The latter model reproduces the differential binding between wild type and the Fc-optimized mAb for human CD16A. The JIMT-1 human breast tumor line, derived from a patient that progressed on trastuzumab therapy, was used in these studies. Single and repeat dose toxicology studies with MGAH22 administered intravenously at high dose were conducted in cynomolgus monkeys.
Results: The optimized Fc domain confers enhanced ADCC against all HER2-positive tumor cells tested, including cells resistant to trastuzumab's anti-proliferative activity or expressing low HER2 levels. The greatest improvement occurs with effector cells isolated from donors homozygous or heterozygous for CD16A-158F, the low-binding allele. MGAH22 demonstrates increased activity against HER2-expressing tumors in mice transgenic for human CD16A-158F. In single and repeat-dose toxicology studies in cynomolgus monkeys, a species with a HER2 expression pattern comparable to that in humans and Fcγ receptors that exhibit enhanced binding to the optimized Fc domain, MGAH22 was well tolerated at all doses tested (15-150 mg/kg) and exhibited pharmacokinetic parameters similar to that of other anti-HER2 antibodies. Induction of cytokine release by MGAH22 in vivo or in vitro was similar to that induced by the corresponding wild type mAb or trastuzumab.
Conclusions: The data support the clinical development of MGAH22, which may have utility in patients with low HER2 expressing tumors or carrying the CD16A low-binding allele.
Figures

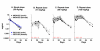
References
-
- Cobleigh MA, Vogel CL, Tripathy D, Robert NJ, Scholl S, Fehrenbacher L, Wolter JM, Paton V, Shak S, Lieberman G, Slamon DJ. Multinational study of the efficacy and safety of humanized anti-HER2 monoclonal antibody in women who have HER2-overexpressing metastatic breast cancer that has progressed after chemotherapy for metastatic disease. J Clin Oncol. 1999;17:2639–2648. - PubMed
-
- Slamon DJ, Leyland-Jones B, Shak S, Fuchs H, Paton V, Bajamonde A, Fleming T, Eiermann W, Wolter J, Pegram M, Baselga J, Norton L. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344:783–792. doi: 10.1056/NEJM200103153441101. - DOI - PubMed
-
- Romond EH, Perez EA, Bryant J, Suman VJ, Geyer CE Jr, Davidson NE, Tan-Chiu E, Martino S, Paik S, Kaufman PA, Swain SM, Pisansky TM, Fehrenbacher L, Kutteh LA, Vogel VG, Visscher DW, Yothers G, Jenkins RB, Brown AM, Dakhil SR, Mamounas EP, Lingle WL, Klein PM, Ingle JN, Wolmark N. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med. 2005;353:1673–1684. doi: 10.1056/NEJMoa052122. - DOI - PubMed
-
- Bang YJ, Van CE, Feyereislova A, Chung HC, Shen L, Sawaki A, Lordick F, Ohtsu A, Omuro Y, Satoh T, Aprile G, Kulikov E, Hill J, Lehle M, Ruschoff J, Kang YK. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet. 2010;376:687–697. doi: 10.1016/S0140-6736(10)61121-X. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

