Expanding the rule set of DNA circuitry with associative toehold activation
- PMID: 22129141
- PMCID: PMC3260326
- DOI: 10.1021/ja206690a
Expanding the rule set of DNA circuitry with associative toehold activation
Abstract
Toehold-mediated strand displacement has proven extremely powerful in programming enzyme-free DNA circuits and DNA nanomachines. To achieve multistep, autonomous, and complex behaviors, toeholds must be initially inactivated by hybridizing to inhibitor strands or domains and then relieved from inactivation in a programmed, timed manner. Although powerful and reasonably robust, this strategy has several drawbacks that limit the architecture of DNA circuits. For example, the combination between toeholds and branch migration (BM) domains is 'hard wired' during DNA synthesis thus cannot be created or changed during the execution of DNA circuits. To solve this problem, I propose a strategy called 'associative toehold activation', where the toeholds and BM domains are connected via hybridization of auxiliary domains during the execution of DNA circuits. Bulged thymidines that stabilize DNA three-way junctions substantially accelerate strand displacement reactions in this scheme, allowing fast strand displacement initiated by reversible toehold binding. To demonstrate the versatility of the scheme, I show (1) run-time combination of toeholds and BM domains, (2) run-time recombination of toeholds and BM domains, which results in a novel operation 'toehold switching', and (3) design of a simple conformational self-replicator.
© 2011 American Chemical Society
Figures
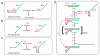
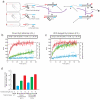

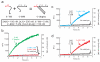
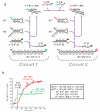
Similar articles
-
Regulation of DNA Strand Displacement Using an Allosteric DNA Toehold.J Am Chem Soc. 2016 Oct 26;138(42):14076-14082. doi: 10.1021/jacs.6b08794. Epub 2016 Oct 13. J Am Chem Soc. 2016. PMID: 27704809
-
Cooperative Toehold: A Mechanism To Activate DNA Strand Displacement and Construct Biosensors.Anal Chem. 2018 Aug 21;90(16):9751-9760. doi: 10.1021/acs.analchem.8b01202. Epub 2018 Aug 3. Anal Chem. 2018. PMID: 30040891
-
Control of DNA strand displacement kinetics using toehold exchange.J Am Chem Soc. 2009 Dec 2;131(47):17303-14. doi: 10.1021/ja906987s. J Am Chem Soc. 2009. PMID: 19894722
-
Principles and Applications of Nucleic Acid Strand Displacement Reactions.Chem Rev. 2019 May 22;119(10):6326-6369. doi: 10.1021/acs.chemrev.8b00580. Epub 2019 Feb 4. Chem Rev. 2019. PMID: 30714375 Review.
-
DNA Strand Displacement Reaction: A Powerful Tool for Discriminating Single Nucleotide Variants.Top Curr Chem (Cham). 2020 Jan 2;378(1):10. doi: 10.1007/s41061-019-0274-z. Top Curr Chem (Cham). 2020. PMID: 31894426 Review.
Cited by
-
DNA branch migration reactions through photocontrollable toehold formation.J Am Chem Soc. 2013 May 29;135(21):7967-73. doi: 10.1021/ja4018495. Epub 2013 May 16. J Am Chem Soc. 2013. PMID: 23642046 Free PMC article.
-
DNA "nano-claw": logic-based autonomous cancer targeting and therapy.J Am Chem Soc. 2014 Jan 29;136(4):1256-9. doi: 10.1021/ja4114903. Epub 2014 Jan 13. J Am Chem Soc. 2014. PMID: 24367989 Free PMC article.
-
Entropy-driven DNA logic circuits regulated by DNAzyme.Nucleic Acids Res. 2018 Sep 19;46(16):8532-8541. doi: 10.1093/nar/gky663. Nucleic Acids Res. 2018. PMID: 30053158 Free PMC article.
-
Leveraging Steric Moieties for Kinetic Control of DNA Strand Displacement Reactions.J Am Chem Soc. 2023 Aug 2;145(30):16691-16703. doi: 10.1021/jacs.3c04344. Epub 2023 Jul 24. J Am Chem Soc. 2023. PMID: 37487322 Free PMC article.
-
On the biophysics and kinetics of toehold-mediated DNA strand displacement.Nucleic Acids Res. 2013 Dec;41(22):10641-58. doi: 10.1093/nar/gkt801. Epub 2013 Sep 9. Nucleic Acids Res. 2013. PMID: 24019238 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

