Effect of pregnancy on emtricitabine pharmacokinetics
- PMID: 22129166
- PMCID: PMC3342997
- DOI: 10.1111/j.1468-1293.2011.00965.x
Effect of pregnancy on emtricitabine pharmacokinetics
Abstract
Objectives: The aim of the study was to describe emtricitabine pharmacokinetics during pregnancy and postpartum.
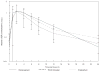
Methods: The International Maternal Pediatric and Adolescent AIDS Clinical Trials (IMPAACT), formerly Pediatric AIDS Clinical Trials Group (PACTG), study P1026s is a prospective pharmacokinetic study of HIV-infected pregnant women taking antiretrovirals for clinical indications, including a cohort taking emtricitabine 200 mg once daily. Intensive steady-state 24-hour emtricitabine pharmacokinetic profiles were performed during the third trimester and 6-12 weeks postpartum, and on maternal and umbilical cord blood samples collected at delivery. Emtricitabine was measured by liquid chromatography-mass spectrometry with a quantification limit of 0.0118 mg/L. The target emtricitabine area under the concentration versus time curve, from time 0 to 24 hours post dose (AUC(0-24) ), was ≥7 mg h/L (≤30% reduction from the typical AUC of 10 mg h/L in nonpregnant historical controls). Third-trimester and postpartum pharmacokinetics were compared within subjects.
Results: Twenty-six women had pharmacokinetics assessed during the third trimester (median 35 weeks of gestation) and 22 postpartum (median 8 weeks postpartum). Mean [90% confidence interval (CI)] emtricitabine pharmacokinetic parameters during the third trimester vs. postpartum were, respectively: AUC: 8.0 (7.1-8.9) vs. 9.7 (8.6-10.9) mg h/L (P = 0.072); apparent clearance (CL/F): 25.0 (22.6-28.3) vs. 20.6 (18.4-23.2) L/h (P = 0.025); 24 hour post dose concentration (C(24) ): 0.058 (0.037-0.063) vs. 0.085 (0.070-0.010) mg/L (P = 0.006). The mean cord:maternal ratio was 1.2 (90% CI 1.0-1.5). The viral load was <400 HIV-1 RNA copies/mL in 24 of 26 women in the third trimester, in 24 of 26 at delivery, and in 15 of 19 postpartum. Within-subject comparisons demonstrated significantly higher CL/F and significantly lower C(24) during pregnancy; however, the C(24) was well above the inhibitory concentration 50%, or drug concentration that suppresses viral replication by half (IC(50) ) in all subjects.
Conclusions: While we found higher emtricitabine CL/F and lower C(24) and AUC during pregnancy compared with postpartum, these changes were not sufficiently large to warrant dose adjustment during pregnancy. Umbilical cord blood concentrations were similar to maternal concentrations.
© 2011 British HIV Association.
Figures


References
-
- Panel on Treatment of HIV-Infected Pregnant Women and Prevention of Perinatal Transmission. [accessed 12 October 2010];Recommendations for Use of Antiretroviral Drugs in Pregnant HIV-1-Infected Women for Maternal Health and Interventions to Reduce Perinatal HIV Transmission in the United States. 2010 May 24;:1–117. Available at http://aidsinfo.nih.gov/ContentFiles/PerinatalGL.pdf.
-
- Mirochnick M, Best BM, Clarke DF. Antiretroviral pharmacology: special issues regarding pregnant women and neonates. Clin Perinatol. 2010;37:907–927. - PubMed
-
- Simone C, Derewlany LO, Koren G. Drug transfer across the placenta. Considerations in treatment and research. Clin Perinatal. 1994;21:463–481. - PubMed
-
- Pacifici GM, Nottoli R. Placental transfer of drugs administered to the mother. Clin Pharmacokinet. 1995;28:235–269. - PubMed
-
- U.S. Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research. [accessed 12 October 2010];Draft guidance for industry: pharmacokinetics in pregnancy-study design, data analysis, and impact on dosing and labeling. 2004 Available at www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guid....
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

