Bridging health technology assessment (HTA) with multicriteria decision analyses (MCDA): field testing of the EVIDEM framework for coverage decisions by a public payer in Canada
- PMID: 22129247
- PMCID: PMC3248909
- DOI: 10.1186/1472-6963-11-329
Bridging health technology assessment (HTA) with multicriteria decision analyses (MCDA): field testing of the EVIDEM framework for coverage decisions by a public payer in Canada
Abstract
Background: Consistent healthcare decision making requires systematic consideration of decision criteria and evidence available to inform them. This can be tackled by combining multicriteria decision analysis (MCDA) and Health Technology Assessment (HTA). The objective of this study was to field-test a decision support framework (EVIDEM), explore its utility to a drug advisory committee and test its reliability over time.
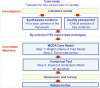
Methods: Tramadol for chronic non-cancer pain was selected by the health plan as a case study relevant to their context. Based on extensive literature review, a by-criterion HTA report was developed to provide synthesized evidence for each criterion of the framework (14 criteria for the MCDA Core Model and 6 qualitative criteria for the Contextual Tool). During workshop sessions, committee members tested the framework in three steps by assigning: 1) weights to each criterion of the MCDA Core Model representing individual perspective; 2) scores for tramadol for each criterion of the MCDA Core Model using synthesized data; and 3) qualitative impacts of criteria of the Contextual Tool on the appraisal. Utility and reliability of the approach were explored through discussion, survey and test-retest. Agreement between test and retest data was analyzed by calculating intra-rater correlation coefficients (ICCs) for weights, scores and MCDA value estimates.
Results: The framework was found useful by the drug advisory committee in supporting systematic consideration of a broad range of criteria to promote a consistent approach to appraising healthcare interventions. Directly integrated in the framework as a "by-criterion" HTA report, synthesized evidence for each criterion facilitated its consideration, although this was sometimes limited by lack of relevant data. Test-retest analysis showed fair to good consistency of weights, scores and MCDA value estimates at the individual level (ICC ranging from 0.676 to 0.698), thus lending some support for the reliability of the approach. Overall, committee members endorsed the inclusion of most framework criteria and revealed important areas of discussion, clarification and adaptation of the framework to the needs of the committee.
Conclusions: By promoting systematic consideration of all decision criteria and the underlying evidence, the framework allows a consistent approach to appraising healthcare interventions. Further testing and validation are needed to advance MCDA approaches in healthcare decisionmaking.
Figures




Similar articles
-
Bridging health technology assessment (HTA) and efficient health care decision making with multicriteria decision analysis (MCDA): applying the EVIDEM framework to medicines appraisal.Med Decis Making. 2012 Mar-Apr;32(2):376-88. doi: 10.1177/0272989X11416870. Epub 2011 Oct 10. Med Decis Making. 2012. PMID: 21987539
-
Field testing of a multicriteria decision analysis (MCDA) framework for coverage of a screening test for cervical cancer in South Africa.Cost Eff Resour Alloc. 2012 Feb 29;10(1):2. doi: 10.1186/1478-7547-10-2. Cost Eff Resour Alloc. 2012. PMID: 22376143 Free PMC article.
-
Multicriteria decision analysis (MCDA) for health technology assessment: the Queensland Health experience.Aust Health Rev. 2019 Oct;43(5):591-599. doi: 10.1071/AH18042. Aust Health Rev. 2019. PMID: 30205873
-
Multicriteria Decision Analysis to Support Health Technology Assessment Agencies: Benefits, Limitations, and the Way Forward.Value Health. 2019 Nov;22(11):1283-1288. doi: 10.1016/j.jval.2019.06.014. Epub 2019 Oct 16. Value Health. 2019. PMID: 31708065
-
Multicriteria decision analysis in health care decision in oncology: a systematic review.Expert Rev Pharmacoecon Outcomes Res. 2022 Apr;22(3):365-380. doi: 10.1080/14737167.2022.2019580. Epub 2021 Dec 27. Expert Rev Pharmacoecon Outcomes Res. 2022. PMID: 34913775
Cited by
-
Early Health Technology Assessment during Nonalcoholic Steatohepatitis Drug Development: A Two-Round, Cross-Country, Multicriteria Decision Analysis.Med Decis Making. 2020 Aug;40(6):830-845. doi: 10.1177/0272989X20940672. Med Decis Making. 2020. PMID: 32845234 Free PMC article.
-
Addressing preference heterogeneity in public health policy by combining Cluster Analysis and Multi-Criteria Decision Analysis: Proof of Method.Health Econ Rev. 2015 May 14;5:10. doi: 10.1186/s13561-015-0048-4. eCollection 2015. Health Econ Rev. 2015. PMID: 25992305 Free PMC article.
-
Multicriteria decision analysis in oncology.Health Expect. 2015 Dec;18(6):1812-26. doi: 10.1111/hex.12178. Epub 2014 Mar 17. Health Expect. 2015. PMID: 24635949 Free PMC article. Review.
-
Balancing costs and benefits at different stages of medical innovation: a systematic review of Multi-criteria decision analysis (MCDA).BMC Health Serv Res. 2015 Jul 9;15:262. doi: 10.1186/s12913-015-0930-0. BMC Health Serv Res. 2015. PMID: 26152122 Free PMC article.
-
Exploring the perspectives and preferences for HTA across German healthcare stakeholders using a multi-criteria assessment of a pulmonary heart sensor as a case study.Health Res Policy Syst. 2015 Apr 28;13:24. doi: 10.1186/s12961-015-0011-1. Health Res Policy Syst. 2015. PMID: 25928535 Free PMC article.
References
-
- Nord E, Daniels N, Kamlet M. QALYs: some challenges. Value Health. 2009;12(Suppl 1):S10–S15. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

