Endothelial follicle stimulating hormone receptor in primary kidney cancer correlates with subsequent response to sunitinib
- PMID: 22129368
- PMCID: PMC3822971
- DOI: 10.1111/j.1582-4934.2011.01495.x
Endothelial follicle stimulating hormone receptor in primary kidney cancer correlates with subsequent response to sunitinib
Abstract
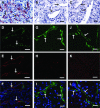
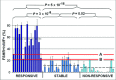
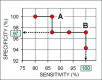
Sunitinib is an anti-angiogenic receptor tyrosine kinase inhibitor used to treat advanced metastatic renal cell carcinoma and other types of cancer. Sutent is effective in only approximately 70% of clear cell renal cell carcinoma (CCRCC) patients, has significant adverse side effects and no method is available to predict which patients will not respond. Our purpose was to explore the possibility of introducing an effective prediction method based on a marker of the tumour vasculature, the follicle stimulating hormone receptor (FSHR). Fifty patients diagnosed with advanced metastatic CCRCC have been subjected to surgery for removal of the primary tumour and were subsequently treated with sunitinib. After three months of therapy the patients were categorized as 'responsive', 'stable' or 'non-responsive' based on the RECIST guidelines. The blood vessel density and the percentage of FSHR-positive vessels were determined by immunofluorescence on sections from the primary tumours removed by surgery, prior to the sunitinib treatment. The percentage of FSHR-stained vessels was on average fivefold higher for the patients who responded to the treatment in comparison with the stable group and almost eightfold higher than in the non-responsive group. The percentage allowed the detection of responders with 87-100% sensitivity and specificity. No significant differences were detected in the total density of vessels among the three groups. The data suggest that FSHR expression levels in the blood vessels of CCRCC primary tumours can be used to predict, with high sensitivity and specificity, the patients who will respond to sunitinib therapy.
© 2012 The Authors Journal of Cellular and Molecular Medicine © 2012 Foundation for Cellular and Molecular Medicine/Blackwell Publishing Ltd.
Figures
Similar articles
-
Analyses of potential predictive markers and survival data for a response to sunitinib in patients with metastatic renal cell carcinoma.PLoS One. 2013 Sep 27;8(9):e76386. doi: 10.1371/journal.pone.0076386. eCollection 2013. PLoS One. 2013. PMID: 24086736 Free PMC article.
-
Active angiogenesis in metastatic renal cell carcinoma predicts clinical benefit to sunitinib-based therapy.Br J Cancer. 2014 May 27;110(11):2700-7. doi: 10.1038/bjc.2014.225. Epub 2014 May 1. Br J Cancer. 2014. PMID: 24786599 Free PMC article.
-
Angiogenic, inflammatory and immunologic markers in predicting response to sunitinib in metastatic renal cell carcinoma.Cancer Sci. 2017 Sep;108(9):1858-1863. doi: 10.1111/cas.13320. Epub 2017 Aug 20. Cancer Sci. 2017. PMID: 28699300 Free PMC article.
-
Sunitinib for the management of advanced renal cell carcinoma.Expert Rev Anticancer Ther. 2010 Mar;10(3):305-17. doi: 10.1586/era.10.26. Expert Rev Anticancer Ther. 2010. PMID: 20214511 Review.
-
Sunitinib (Sutent): a novel agent for the treatment of metastatic renal cell carcinoma.J Oncol Pharm Pract. 2007 Mar;13(1):5-15. doi: 10.1177/1078155207077924. J Oncol Pharm Pract. 2007. PMID: 17621562 Review.
Cited by
-
PET Imaging of FSHR Expression in Tumors with 68Ga-Labeled FSH1 Peptide.Contrast Media Mol Imaging. 2017 Aug 23;2017:2674502. doi: 10.1155/2017/2674502. eCollection 2017. Contrast Media Mol Imaging. 2017. PMID: 29097913 Free PMC article.
-
Revisiting the expression and function of follicle-stimulation hormone receptor in human umbilical vein endothelial cells.Sci Rep. 2016 Nov 16;6:37095. doi: 10.1038/srep37095. Sci Rep. 2016. PMID: 27848975 Free PMC article.
-
Pilot study of a novel (18)F-labeled FSHR probe for tumor imaging.Mol Imaging Biol. 2014 Aug;16(4):578-85. doi: 10.1007/s11307-013-0712-1. Mol Imaging Biol. 2014. PMID: 24389931
-
Follicle-stimulating hormone promotes renal tubulointerstitial fibrosis in aging women via the AKT/GSK-3β/β-catenin pathway.Aging Cell. 2019 Oct;18(5):e12997. doi: 10.1111/acel.12997. Epub 2019 Jun 26. Aging Cell. 2019. PMID: 31243899 Free PMC article.
-
Antibody Selection for Cancer Target Validation of FSH-Receptor in Immunohistochemical Settings.Antibodies (Basel). 2017 Oct 18;6(4):15. doi: 10.3390/antib6040015. Antibodies (Basel). 2017. PMID: 31548530 Free PMC article.
References
-
- Jemal A, Siegel R, Xu J, et al. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. - PubMed
-
- Kontovinis LF, Papazisis KT, Touplikioti P, et al. Sunitinib treatment for patients with clear-cell metastatic renal cell carcinoma: clinical outcomes and plasma angiogenesis markers. BMC Cancer. 2009;9:82. doi: 10.1186/1471-2407-9-82. - DOI - PMC - PubMed
-
- Mena AC, Pulido EG, Guillén-Ponce C. Understanding the molecular-based mechanism of action of the tyrosine kinase inhibitor: sunitinib. Anticancer Drugs. 2010;21:S3–11. - PubMed
-
- Adams VR, Leggas M. Sunitinib malate for the treatment of metastatic renal cell carcinoma and gastrointestinal stromal tumours. Clin Ther. 2007;29:1338–53. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

