13-hydroxy linoleic acid increases expression of the cholesterol transporters ABCA1, ABCG1 and SR-BI and stimulates apoA-I-dependent cholesterol efflux in RAW264.7 macrophages
- PMID: 22129452
- PMCID: PMC3248880
- DOI: 10.1186/1476-511X-10-222
13-hydroxy linoleic acid increases expression of the cholesterol transporters ABCA1, ABCG1 and SR-BI and stimulates apoA-I-dependent cholesterol efflux in RAW264.7 macrophages
Abstract
Background: Synthetic activators of peroxisome proliferator-activated receptors (PPARs) stimulate cholesterol removal from macrophages through PPAR-dependent up-regulation of liver × receptor α (LXRα) and subsequent induction of cholesterol exporters such as ATP-binding cassette transporter A1 (ABCA1) and scavenger receptor class B type 1 (SR-BI). The present study aimed to test the hypothesis that the hydroxylated derivative of linoleic acid (LA), 13-HODE, which is a natural PPAR agonist, has similar effects in RAW264.7 macrophages.
Methods: RAW264.7 macrophages were treated without (control) or with LA or 13-HODE in the presence and absence of PPARα or PPARγ antagonists and determined protein levels of LXRα, ABCA1, ABCG1, SR-BI, PPARα and PPARγ and apolipoprotein A-I mediated lipid efflux.
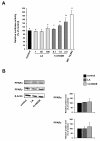
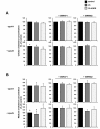
Results: Treatment of RAW264.7 cells with 13-HODE increased PPAR-transactivation activity and protein concentrations of LXRα, ABCA1, ABCG1 and SR-BI when compared to control treatment (P < 0.05). In addition, 13-HODE enhanced cholesterol concentration in the medium but decreased cellular cholesterol concentration during incubation of cells with the extracellular lipid acceptor apolipoprotein A-I (P < 0.05). Pre-treatment of cells with a selective PPARα or PPARγ antagonist completely abolished the effects of 13-HODE on cholesterol efflux and protein levels of genes investigated. In contrast to 13-HODE, LA had no effect on either of these parameters compared to control cells.
Conclusion: 13-HODE induces cholesterol efflux from macrophages via the PPAR-LXRα-ABCA1/SR-BI-pathway.
Figures

Similar articles
-
Propofol up-regulates expression of ABCA1, ABCG1, and SR-B1 through the PPARγ/LXRα signaling pathway in THP-1 macrophage-derived foam cells.Cardiovasc Pathol. 2015 Jul-Aug;24(4):230-5. doi: 10.1016/j.carpath.2014.12.004. Epub 2014 Dec 27. Cardiovasc Pathol. 2015. PMID: 25600616
-
Lipopolysaccharide represses the expression of ATP-binding cassette transporter G1 and scavenger receptor class B, type I in murine macrophages.Inflamm Res. 2012 May;61(5):465-72. doi: 10.1007/s00011-011-0433-3. Epub 2012 Jan 13. Inflamm Res. 2012. PMID: 22240665
-
Telmisartan enhances cholesterol efflux from THP-1 macrophages by activating PPARgamma.J Atheroscler Thromb. 2007 Jun;14(3):133-41. doi: 10.5551/jat.14.133. J Atheroscler Thromb. 2007. PMID: 17587765
-
Foam cells in atherosclerosis.Clin Chim Acta. 2013 Sep 23;424:245-52. doi: 10.1016/j.cca.2013.06.006. Epub 2013 Jun 16. Clin Chim Acta. 2013. PMID: 23782937 Review.
-
Different Pathways of Cellular Cholesterol Efflux.Cell Biochem Biophys. 2022 Sep;80(3):471-481. doi: 10.1007/s12013-022-01081-5. Epub 2022 Jun 23. Cell Biochem Biophys. 2022. PMID: 35737216 Review.
Cited by
-
Naoxintong Retards Atherosclerosis by Inhibiting Foam Cell Formation Through Activating Pparα Pathway.Curr Mol Med. 2018;18(10):698-710. doi: 10.2174/1566524019666190207143207. Curr Mol Med. 2018. PMID: 30734676 Free PMC article.
-
Fatty Acids in Childhood Obesity: A Link Between Nutrition, Metabolic Alterations and Cardiovascular Risk.J Lipid Atheroscler. 2025 May;14(2):200-218. doi: 10.12997/jla.2025.14.2.200. Epub 2025 Apr 3. J Lipid Atheroscler. 2025. PMID: 40492180 Free PMC article.
-
Recombinant Humanized IgG1 Antibody Promotes Reverse Cholesterol Transport through FcRn-ERK1/2-PPARα Pathway in Hepatocytes.Int J Mol Sci. 2022 Nov 23;23(23):14607. doi: 10.3390/ijms232314607. Int J Mol Sci. 2022. PMID: 36498935 Free PMC article.
-
Macrophage-Mediated Immune Responses: From Fatty Acids to Oxylipins.Molecules. 2021 Dec 28;27(1):152. doi: 10.3390/molecules27010152. Molecules. 2021. PMID: 35011385 Free PMC article. Review.
-
Vitamin B12 coordinates ileal epithelial cell and microbiota functions to resist Salmonella infection in mice.J Exp Med. 2022 Jul 4;219(7):e20220057. doi: 10.1084/jem.20220057. Epub 2022 Jun 8. J Exp Med. 2022. PMID: 35674742 Free PMC article.
References
-
- Liu JF, Huang CJ. Tissue α-tocopherol retention in male rats is compromised by feeding diets containing oxidized frying oil. J Nutr. 1995;125:3071–3080. - PubMed
-
- Eder K, Keller U, Hirche F, Brandsch C. Thermally oxidized dietary fats increase the susceptibility of rat LDL to lipid peroxidation but not their uptake by macrophages. J Nutr. 2003;133:2830–2837. - PubMed
-
- Chao PM, Chao CY, Lin FJ, Huang CJ. Oxidized frying oil up-regulates hepatic acyl-CoA oxidase and cytochrome P450 4A1 genes in rats and activates PPARα. J Nutr. 2001;131:3166–3174. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

