Soluble thrombomodulin reduces inflammation and prevents microalbuminuria induced by chronic endothelial activation in transgenic mice
- PMID: 22129968
- PMCID: PMC3311320
- DOI: 10.1152/ajprenal.00558.2011
Soluble thrombomodulin reduces inflammation and prevents microalbuminuria induced by chronic endothelial activation in transgenic mice
Abstract
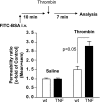
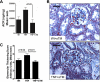
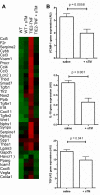
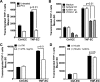
Chronic kidney disease pathogenesis involves both tubular and vascular injuries. Despite abundant investigations to identify the risk factors, the involvement of chronic endothelial dysfunction in developing nephropathies is insufficiently explored. Previously, soluble thrombomodulin (sTM), a cofactor in the activation of protein C, has been shown to protect endothelial function in models of acute kidney injury. In this study, the role for sTM in treating chronic kidney disease was explored by employing a mouse model of chronic vascular activation using endothelial-specific TNF-α-expressing (tie2-TNF) mice. Analysis of kidneys from these mice after 3 mo showed no apparent phenotype, whereas 6-mo-old mice demonstrated infiltration of CD45-positive leukocytes accompanied by upregulated gene expression of inflammatory chemokines, markers of kidney injury, and albuminuria. Intervention with murine sTM with biweekly subcutaneous injections during this window of disease development between months 3 and 6 prevented the development of kidney pathology. To better understand the mechanisms of these findings, we determined whether sTM could also prevent chronic endothelial cell activation in vitro. Indeed, treatment with sTM normalized increased chemokines, adhesion molecule expression, and reduced transmigration of monocytes in continuously activated TNF-expressing endothelial cells. Our results suggest that vascular inflammation associated with vulnerable endothelium can contribute to loss in renal function as suggested by the tie2-TNF mice, a unique model for studying the role of vascular activation and inflammation in chronic kidney disease. Furthermore, the ability to restore the endothelial balance by exogenous administration of sTM via downregulation of specific adhesion molecules and chemokines suggests a potential for therapeutic intervention in kidney disease associated with chronic inflammation.
Figures




Similar articles
-
Chronic inflammation and protection from acute hepatitis in transgenic mice expressing TNF in endothelial cells.J Immunol. 2001 Oct 1;167(7):3944-52. doi: 10.4049/jimmunol.167.7.3944. J Immunol. 2001. PMID: 11564813
-
Shock-induced stress induces loss of microvascular endothelial Tie2 in the kidney which is not associated with reduced glomerular barrier function.Am J Physiol Renal Physiol. 2009 Aug;297(2):F272-81. doi: 10.1152/ajprenal.00137.2009. Epub 2009 Jun 10. Am J Physiol Renal Physiol. 2009. PMID: 19515812
-
Endothelial injury is closely related to osteopontin and TNF receptor-mediated inflammation in end-stage renal disease.Cytokine. 2019 Sep;121:154729. doi: 10.1016/j.cyto.2019.05.016. Epub 2019 May 30. Cytokine. 2019. PMID: 31153055
-
Thrombomodulin as a Physiological Modulator of Intravascular Injury.Front Immunol. 2020 Sep 16;11:575890. doi: 10.3389/fimmu.2020.575890. eCollection 2020. Front Immunol. 2020. PMID: 33042158 Free PMC article. Review.
-
Endothelial dysfunction as an underlying pathophysiological condition of chronic kidney disease.Clin Exp Nephrol. 2012 Aug;16(4):518-21. doi: 10.1007/s10157-012-0646-y. Epub 2012 Jun 6. Clin Exp Nephrol. 2012. PMID: 22669535 Review.
Cited by
-
Featured Article: Deterioration of visual function mediated by senescence-associated endoplasmic reticulum stress in inflammatory tie2-TNF mice.Exp Biol Med (Maywood). 2018 Aug;243(12):976-984. doi: 10.1177/1535370218794915. Epub 2018 Aug 16. Exp Biol Med (Maywood). 2018. PMID: 30114984 Free PMC article.
-
The emerging role of coagulation proteases in kidney disease.Nat Rev Nephrol. 2016 Feb;12(2):94-109. doi: 10.1038/nrneph.2015.177. Epub 2015 Nov 23. Nat Rev Nephrol. 2016. PMID: 26592189 Free PMC article. Review.
-
GRP78 translocation to the cell surface and O-GlcNAcylation of VE-Cadherin contribute to ER stress-mediated endothelial permeability.Sci Rep. 2019 Jul 25;9(1):10783. doi: 10.1038/s41598-019-47246-w. Sci Rep. 2019. PMID: 31346222 Free PMC article.
-
Cysteine Leukotriene Receptor Antagonist-Montelukast Effects on Diabetic Retinal Microvascular Endothelial Cells Curtail Autophagy.Invest Ophthalmol Vis Sci. 2024 Nov 4;65(13):15. doi: 10.1167/iovs.65.13.15. Invest Ophthalmol Vis Sci. 2024. PMID: 39504050 Free PMC article.
-
The involvement of endothelial mediators in leprosy.Mem Inst Oswaldo Cruz. 2016 Oct;111(10):635-641. doi: 10.1590/0074-02760160122. Epub 2016 Oct 3. Mem Inst Oswaldo Cruz. 2016. PMID: 27706378 Free PMC article.
References
-
- Basile DP. The endothelial cell in ischemic acute kidney injury: implications for acute and chronic function. Kidney Int 72: 151–156, 2007 - PubMed
-
- Basile DP, Friedrich JL, Spahic J, Knipe N, Mang H, Leonard EC, Changizi-Ashtiyani S, Bacallao RL, Molitoris BA, Sutton TA. Impaired endothelial proliferation and mesenchymal transition contribute to vascular rarefaction following acute kidney injury. Am J Physiol Renal Physiol 300: F721–F733, 2011 - PMC - PubMed
-
- Boehme MW, Nawroth PP, Kling E, Lin J, Amiral J, Riedesel J, Raeth U, Scherbaum WA. Serum thrombomodulin. A novel marker of disease activity in systemic lupus erythematosus. Arthritis Rheum 37: 572–577, 1994 - PubMed
-
- Budhiraja S, Singh J. Protein kinase C beta inhibitors: a new therapeutic target for diabetic nephropathy and vascular complications. Fundam Clin Pharmacol 22: 231–240, 2008 - PubMed
-
- Caramori ML, Fioretto P, Mauer M. The need for early predictors of diabetic nephropathy risk: is albumin excretion rate sufficient? Diabetes 49: 1399–1408, 2000 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

