The effects of extended pre-quit varenicline treatment on smoking behavior and short-term abstinence: a randomized clinical trial
- PMID: 22130118
- PMCID: PMC3325094
- DOI: 10.1038/clpt.2011.317
The effects of extended pre-quit varenicline treatment on smoking behavior and short-term abstinence: a randomized clinical trial
Abstract
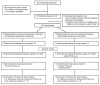
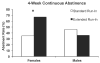
Preclinical research and learning theory suggest that a longer duration of varenicline treatment prior to the target quit date (TQD) would reduce smoking rates before cessation and improve abstinence outcomes. A double-blind randomized controlled trial tested this hypothesis in 60 smokers randomized to either an Extended run-in group (4 weeks of pre-TQD varenicline) or a Standard run-in group (3 weeks of placebo, 1 week of pre-TQD varenicline); all the participants received 11 weeks of post-TQD varenicline and brief counseling. During the pre-quit run-in, the reduction in smoking rates was greater in the Extended run-in group than in the Standard run-in group (42% vs. 24%, P < 0.01), and this effect was greater in women than in men (57% vs. 26%, P = 0.001). The rate of continuous abstinence during the final 4 weeks of treatment was higher among women in the Extended group compared to women in the Standard run-in group (67% vs. 35%). Although these data suggest that extension of varenicline treatment reduces smoking during the pre-quit period and may further enhance cessation rates, confirmatory evidence is needed from phase III clinical trials.
Figures

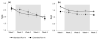
References
-
- Center for Disease Control . MMWR Morb Mort Wkly Rep. Vol. 57. 2008. Cigarette smoking among adults - United States, 2007; pp. 1221–6. - PubMed
-
- AFiore MC, Jaen CR, Baker TB, et al. Clinical Practice Guideline. U.S. Department of Health and Human Service. Public Health Service; Rockville, MD: 2008. Treating Tobacco Use and Dependence: 2008 Update.
-
- Cahill K, Stead LF, Lancaster T. Nicotine receptor partial agonists for smoking cessation. Cochrane Database Syst Rev. 2011;2:CD006103. - PubMed
-
- Lerman C, et al. Translational research in medication development for nicotine dependence. Nat Rev Drug Discov. 2007;6:746–62. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

