A PLA1-2 punch regulates the Golgi complex
- PMID: 22130221
- PMCID: PMC3273632
- DOI: 10.1016/j.tcb.2011.10.003
A PLA1-2 punch regulates the Golgi complex
Abstract
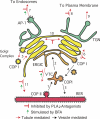
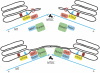
The mammalian Golgi complex, trans Golgi network (TGN) and ER-Golgi intermediate compartment (ERGIC) are comprised of membrane cisternae, coated vesicles and membrane tubules, all of which contribute to membrane trafficking and maintenance of their unique architectures. Recently, a new cast of players was discovered to regulate the Golgi and ERGIC: four unrelated cytoplasmic phospholipase A (PLA) enzymes, cPLA(2)α (GIVA cPLA(2)), PAFAH Ib (GVIII PLA(2)), iPLA(2)-β (GVIA-2 iPLA(2)) and iPLA(1)γ. These ubiquitously expressed enzymes regulate membrane trafficking from specific Golgi subcompartments, although there is evidence for some functional redundancy between PAFAH Ib and cPLA(2)α. Three of these enzymes, PAFAH Ib, cPLA(2)α and iPLA(2)-β, exert effects on Golgi structure and function by inducing the formation of membrane tubules. We review our current understanding of how PLA enzymes regulate Golgi and ERGIC morphology and function.
Copyright © 2011 Elsevier Ltd. All rights reserved.
Figures


References
-
- Trucco A, et al. Secretory traffic triggers the formation of tubular continuities across Golgi sub-compartments. Nat Cell Biol. 2004;6:1071–1081. - PubMed
-
- Brown WJ, et al. Phospholipase A2 (PLA2) enzymes in membrane trafficking: mediators of membrane shape and function. Traffic. 2003;4:214–221. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

