The safety and efficacy of sublingual and oral immunotherapy for milk allergy
- PMID: 22130425
- PMCID: PMC3437605
- DOI: 10.1016/j.jaci.2011.10.023
The safety and efficacy of sublingual and oral immunotherapy for milk allergy
Abstract
Background: Oral immunotherapy (OIT) and sublingual immunotherapy (SLIT) are potential therapies for food allergy, but the optimal method of administration, mechanism of action, and duration of response remain unknown.
Objective: We sought to explore the safety and efficacy of OIT and SLIT for the treatment of cow's milk (CM) allergy.
Methods: We randomized children with CM allergy to SLIT alone or SLIT followed by OIT. After screening double-blind, placebo-controlled food challenges and initial SLIT escalation, subjects either continued SLIT escalation to 7 mg daily or began OIT to either 1000 mg (the OITB group) or 2000 mg (the OITA group) of milk protein. They were challenged with 8 g of milk protein after 12 and 60 weeks of maintenance. If they passed the 60-week challenge, therapy was withdrawn, with challenges repeated 1 and 6 weeks later. Mechanistic correlates included end point titration skin prick testing and measurement of CM-specific IgE and IgG(4) levels, basophil histamine release, constitutive CD63 expression, CD203c expression, and intracellular spleen tyrosine kinase levels.
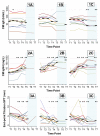
Results: Thirty subjects with CM allergy aged 6 to 17 years were enrolled. After therapy, 1 of 10 subjects in the SLIT group, 6 of 10 subjects in the SLIT/OITB group, and 8 of 10 subjects in the OITA group passed the 8-g challenge (P = .002, SLIT vs OIT). After avoidance, 6 of 15 subjects (3 of 6 subjects in the OITB group and 3 of 8 subjects in the OITA group) regained reactivity, 2 after only 1 week. Although the overall reaction rate was similar, systemic reactions were more common during OIT than during SLIT. By the end of therapy, titrated CM skin prick test results and CD63 and CD203c expression decreased and CM-specific IgG(4) levels increased in all groups, whereas CM-specific IgE and spontaneous histamine release values decreased in only the OIT group.
Conclusion: OIT was more efficacious for desensitization to CM than SLIT alone but was accompanied by more systemic side effects. Clinical desensitization was lost in some cases within 1 week off therapy.
Copyright © 2011 American Academy of Allergy, Asthma & Immunology. Published by Mosby, Inc. All rights reserved.
Figures



Similar articles
-
A randomized, double-blind, placebo-controlled study of milk oral immunotherapy for cow's milk allergy.J Allergy Clin Immunol. 2008 Dec;122(6):1154-60. doi: 10.1016/j.jaci.2008.09.030. Epub 2008 Oct 25. J Allergy Clin Immunol. 2008. PMID: 18951617 Free PMC article. Clinical Trial.
-
Mechanistic correlates of clinical responses to omalizumab in the setting of oral immunotherapy for milk allergy.J Allergy Clin Immunol. 2017 Oct;140(4):1043-1053.e8. doi: 10.1016/j.jaci.2017.03.028. Epub 2017 Apr 13. J Allergy Clin Immunol. 2017. PMID: 28414061 Free PMC article. Clinical Trial.
-
A randomized, double-blind, placebo-controlled pilot study of sublingual versus oral immunotherapy for the treatment of peanut allergy.J Allergy Clin Immunol. 2015 May;135(5):1275-82.e1-6. doi: 10.1016/j.jaci.2014.11.005. Epub 2014 Dec 18. J Allergy Clin Immunol. 2015. PMID: 25528358 Free PMC article. Clinical Trial.
-
Oral and Sublingual Immunotherapy for Treatment of IgE-Mediated Food Allergy.Clin Rev Allergy Immunol. 2018 Oct;55(2):139-152. doi: 10.1007/s12016-018-8677-0. Clin Rev Allergy Immunol. 2018. PMID: 29656306 Review.
-
Development of natural tolerance and induced desensitization in cow's milk allergy.Pediatr Allergy Immunol. 2013 Mar;24(2):114-21. doi: 10.1111/pai.12004. Epub 2012 Sep 9. Pediatr Allergy Immunol. 2013. PMID: 22957704 Review.
Cited by
-
Research Progress in Atopic March.Front Immunol. 2020 Aug 27;11:1907. doi: 10.3389/fimmu.2020.01907. eCollection 2020. Front Immunol. 2020. PMID: 32973790 Free PMC article. Review.
-
Oral immunotherapy for food allergy: towards a new horizon.Allergy Asthma Immunol Res. 2013 Jan;5(1):3-15. doi: 10.4168/aair.2013.5.1.3. Epub 2012 Nov 28. Allergy Asthma Immunol Res. 2013. PMID: 23277873 Free PMC article.
-
Immunological mechanisms for desensitization and tolerance in food allergy.Semin Immunopathol. 2012 Sep;34(5):689-702. doi: 10.1007/s00281-012-0333-9. Epub 2012 Jul 21. Semin Immunopathol. 2012. PMID: 22821087 Free PMC article. Review.
-
Oral Immunotherapy for Children with Cow's Milk Allergy.Pathogens. 2021 Oct 15;10(10):1328. doi: 10.3390/pathogens10101328. Pathogens. 2021. PMID: 34684278 Free PMC article. Review.
-
Allergen-Specific Immunotherapies for Food Allergy.Allergy Asthma Immunol Res. 2018 May;10(3):189-206. doi: 10.4168/aair.2018.10.3.189. Allergy Asthma Immunol Res. 2018. PMID: 29676066 Free PMC article. Review.
References
-
- Nelson HS, Lahr J, Rule R, Bock A, Leung D. Treatment of anaphylactic sensitivity to peanuts by immunotherapy with injections of aqueous peanut extract. J Allergy Clin Immunol. 1997;99:744–51. - PubMed
-
- Allam JP, Stojanovski G, Friedrichs N, Peng W, Bieber T, Wenzel J, et al. Distribution of Langerhans cells and mast cells within the human oral mucosa: new application sites of allergens in sublingual immunotherapy? Allergy. 2008;63:720–7. - PubMed
-
- Enrique E, Pineda F, Malek T, Bartra J, Basagana M, Tella R, et al. Sublingual immunotherapy for hazelnut food allergy: a randomized, double-blind, placebo-controlled study with a standardized hazelnut extract. J Allergy Clin Immunol. 2005;116:1073–9. - PubMed
-
- de Boissieu D, Dupont C. Sublingual immunotherapy for cow’s milk protein allergy: a preliminary report. Allergy. 2006;61:1238–9. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- UL1RR025005/RR/NCRR NIH HHS/United States
- 1KL2RR025006-01/RR/NCRR NIH HHS/United States
- UL1 RR024128/RR/NCRR NIH HHS/United States
- T32 AI007007/AI/NIAID NIH HHS/United States
- KL2 RR025006/RR/NCRR NIH HHS/United States
- K23AI091869/AI/NIAID NIH HHS/United States
- 5T32-AI07007/AI/NIAID NIH HHS/United States
- L30 AI079959/AI/NIAID NIH HHS/United States
- UL1RR024128/RR/NCRR NIH HHS/United States
- UL1 RR025005/RR/NCRR NIH HHS/United States
- T32 AI007062/AI/NIAID NIH HHS/United States
- R21 AI079853/AI/NIAID NIH HHS/United States
- R21AI079853/AI/NIAID NIH HHS/United States
- K23 AI091869/AI/NIAID NIH HHS/United States
- K23 AI103187/AI/NIAID NIH HHS/United States
- 5T32-AI007062-3/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

