The tetherin/BST-2 coiled-coil ectodomain mediates plasma membrane microdomain localization and restriction of particle release
- PMID: 22130541
- PMCID: PMC3302402
- DOI: 10.1128/JVI.05906-11
The tetherin/BST-2 coiled-coil ectodomain mediates plasma membrane microdomain localization and restriction of particle release
Abstract
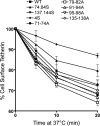
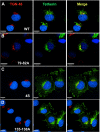
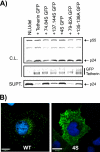
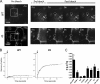
Tetherin/BST-2 forms a proteinaceous tether that restricts the release of a number of enveloped viruses following viral budding. Tetherin is an unusual membrane glycoprotein with two membrane anchors and an extended coiled-coil ectodomain. The ectodomain itself forms an imperfect coil that may undergo conformational shifts to accommodate membrane dynamics during the budding process. The coiled-coil ectodomain is required for restriction, but precisely how it contributes to the restriction of particle release remains under investigation. In this study, mutagenesis of the ectodomain was used to further define the role of the coiled-coil ectodomain in restriction. Scanning mutagenesis throughout much of the ectodomain failed to disrupt the ability of tetherin to restrict HIV particle release, indicating a high degree of plasticity. Targeted N- and C-terminal substitutions disrupting the coiled coil led to both a loss of restriction and an alteration of subcellular distribution. Two ectodomain mutants deficient in restriction were endocytosed inefficiently, and the levels of these mutants on the cell surface were significantly enhanced. An ectodomain mutant with four targeted serine substitutions (4S) failed to cluster in membrane microdomains, was deficient in restriction of particle release, and exhibited an increase in lateral mobility on the membrane. These results suggest that the tetherin ectodomain contributes to microdomain localization and to constrained lateral mobility. We propose that focal clustering of tetherin via ectodomain interactions plays a role in restriction of particle release.
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

