A computational model for the influence of corollary discharge and proprioception on the perisaccadic mislocalization of briefly presented stimuli in complete darkness
- PMID: 22131401
- PMCID: PMC6623809
- DOI: 10.1523/JNEUROSCI.3407-11.2011
A computational model for the influence of corollary discharge and proprioception on the perisaccadic mislocalization of briefly presented stimuli in complete darkness
Abstract
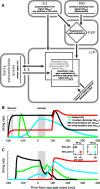
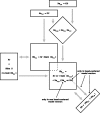
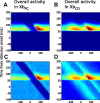
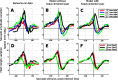
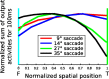
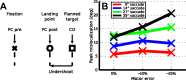
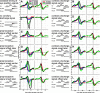
Spatial perception, the localization of stimuli in space, can rely on visual reference stimuli or on egocentric factors such as a stimulus position relative to eye gaze. In total darkness, only an egocentric reference frame provides sufficient information. When stimuli are briefly flashed around saccades, the localization error reveals potential mechanisms of updating such reference frames as described in several theories and computational models. Recent novel experimental evidence, however, showed that the maximum amount of mislocalization does not scale linearly with saccade amplitude but rather stays below 13° even for long saccades, which is different from predicted by present models. We propose a new model of perisaccadic mislocalization in complete darkness to account for this observation. According to this model, mislocalization arises not on the motor side by comparing a retinal position signal with an extraretinal eye position related signal but by updating stimulus position in visual areas through a combination of proprioceptive eye position and corollary discharge. Simulations with realistic input signals and temporal dynamics show that both signals together are used for spatial updating and in turn bring about perisaccadic mislocalization.
Figures





Similar articles
-
Mislocalization of perceived saccade target position induced by perisaccadic visual stimulation.J Neurosci. 2006 Jan 4;26(1):12-20. doi: 10.1523/JNEUROSCI.2407-05.2006. J Neurosci. 2006. PMID: 16399668 Free PMC article.
-
Perisaccadic mislocalization without saccadic eye movements.Neuroscience. 2006 Feb;137(3):737-45. doi: 10.1016/j.neuroscience.2005.09.032. Epub 2005 Nov 14. Neuroscience. 2006. PMID: 16289834 Clinical Trial.
-
Spatial updating of attention across eye movements: A neuro-computational approach.J Vis. 2019 Jul 1;19(7):10. doi: 10.1167/19.7.10. J Vis. 2019. PMID: 31323096
-
Eye proprioception may provide real time eye position information.Neurol Sci. 2013 Mar;34(3):281-6. doi: 10.1007/s10072-012-1172-0. Epub 2012 Aug 8. Neurol Sci. 2013. PMID: 22872063 Review.
-
Corollary Discharge and Oculomotor Proprioception: Cortical Mechanisms for Spatially Accurate Vision.Annu Rev Vis Sci. 2016 Oct 14;2:61-84. doi: 10.1146/annurev-vision-082114-035407. Epub 2016 Aug 19. Annu Rev Vis Sci. 2016. PMID: 28532350 Free PMC article. Review.
Cited by
-
Visuomotor learning from postdictive motor error.Elife. 2021 Mar 9;10:e64278. doi: 10.7554/eLife.64278. Elife. 2021. PMID: 33687328 Free PMC article.
-
Brain circuits underlying visual stability across eye movements-converging evidence for a neuro-computational model of area LIP.Front Comput Neurosci. 2014 Mar 11;8:25. doi: 10.3389/fncom.2014.00025. eCollection 2014. Front Comput Neurosci. 2014. PMID: 24653691 Free PMC article.
-
Bridging Conflicting Views on Eye Position Signals: A Neurocomputational Approach to Perisaccadic Perception: Eye Position Information in Brain and Model.Eur J Neurosci. 2025 Aug;62(3):e70207. doi: 10.1111/ejn.70207. Eur J Neurosci. 2025. PMID: 40758302 Free PMC article.
-
A sensory memory to preserve visual representations across eye movements.Nat Commun. 2021 Nov 8;12(1):6449. doi: 10.1038/s41467-021-26756-0. Nat Commun. 2021. PMID: 34750376 Free PMC article.
-
Compression of Space for Low Visibility Probes.Front Syst Neurosci. 2016 Mar 10;10:21. doi: 10.3389/fnsys.2016.00021. eCollection 2016. Front Syst Neurosci. 2016. PMID: 27013989 Free PMC article.
References
-
- Bremmer F, Distler C, Hoffmann KP. Eye position effects in monkey cortex. II. Pursuit- and fixation-related activity in posterior parietal areas LIP and 7A. J Neurophysiol. 1997;77:962–977. - PubMed
-
- Colby CL, Duhamel JR, Goldberg ME. Visual, presaccadic, and cognitive activation of single neurons in monkey lateral intraparietal area. J Neurophysiol. 1996;76:2841–2852. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
