Influence of static eye and head position on tone-evoked gaze shifts
- PMID: 22131411
- PMCID: PMC6623806
- DOI: 10.1523/JNEUROSCI.5030-10.2011
Influence of static eye and head position on tone-evoked gaze shifts
Abstract
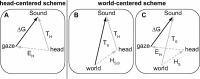
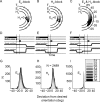
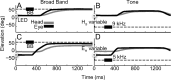
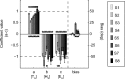
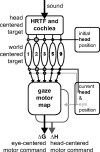
The auditory system represents sound-source directions initially in head-centered coordinates. To program eye-head gaze shifts to sounds, the orientation of eyes and head should be incorporated to specify the target relative to the eyes. Here we test (1) whether this transformation involves a stage in which sounds are represented in a world- or a head-centered reference frame, and (2) whether acoustic spatial updating occurs at a topographically organized motor level representing gaze shifts, or within the tonotopically organized auditory system. Human listeners generated head-unrestrained gaze shifts from a large range of initial eye and head positions toward brief broadband sound bursts, and to tones at different center frequencies, presented in the midsagittal plane. Tones were heard at a fixed illusory elevation, regardless of their actual location, that depended in an idiosyncratic way on initial head and eye position, as well as on the tone's frequency. Gaze shifts to broadband sounds were accurate, fully incorporating initial eye and head positions. The results support the hypothesis that the auditory system represents sounds in a supramodal reference frame, and that signals about eye and head orientation are incorporated at a tonotopic stage.
Figures


Similar articles
-
Influence of head position on the spatial representation of acoustic targets.J Neurophysiol. 1999 Jun;81(6):2720-36. doi: 10.1152/jn.1999.81.6.2720. J Neurophysiol. 1999. PMID: 10368392
-
Dynamic sound localization during rapid eye-head gaze shifts.J Neurosci. 2004 Oct 20;24(42):9291-302. doi: 10.1523/JNEUROSCI.2671-04.2004. J Neurosci. 2004. PMID: 15496665 Free PMC article.
-
Combined eye-head gaze shifts produced by electrical stimulation of the superior colliculus in rhesus monkeys.J Neurophysiol. 1996 Aug;76(2):927-52. doi: 10.1152/jn.1996.76.2.927. J Neurophysiol. 1996. PMID: 8871209
-
On the feedback control of orienting gaze shifts made with eye and head movements.Prog Brain Res. 2003;142:55-68. doi: 10.1016/S0079-6123(03)42006-2. Prog Brain Res. 2003. PMID: 12693254 Review.
-
Neural control of 3-D gaze shifts in the primate.Prog Brain Res. 2003;142:109-24. doi: 10.1016/s0079-6123(03)42009-8. Prog Brain Res. 2003. PMID: 12693257 Review.
Cited by
-
Spatial localization of auditory stimuli in human auditory cortex is based on both head-independent and head-centered coordinate systems.J Neurosci. 2012 Sep 26;32(39):13501-9. doi: 10.1523/JNEUROSCI.1315-12.2012. J Neurosci. 2012. PMID: 23015439 Free PMC article.
-
Audio-Visual Integration in a Redundant Target Paradigm: A Comparison between Rhesus Macaque and Man.Front Syst Neurosci. 2017 Nov 29;11:89. doi: 10.3389/fnsys.2017.00089. eCollection 2017. Front Syst Neurosci. 2017. PMID: 29238295 Free PMC article.
-
Sound Localization in Real-Time Vocoded Cochlear-Implant Simulations With Normal-Hearing Listeners.Trends Hear. 2019 Jan-Dec;23:2331216519847332. doi: 10.1177/2331216519847332. Trends Hear. 2019. PMID: 31088265 Free PMC article.
-
Sounds are remapped across saccades.Sci Rep. 2020 Dec 7;10(1):21332. doi: 10.1038/s41598-020-78163-y. Sci Rep. 2020. PMID: 33288778 Free PMC article.
-
Dependence of auditory spatial updating on vestibular, proprioceptive, and efference copy signals.J Neurophysiol. 2016 Aug 1;116(2):765-75. doi: 10.1152/jn.00052.2016. Epub 2016 May 11. J Neurophysiol. 2016. PMID: 27169504 Free PMC article.
References
-
- Blauert J. Spatial hearing: the psychophysics of human sound localization. Cambridge, MA: MIT Press; 1997. Revised edition.
-
- Chen LL. Head movements evoked by electrical stimulation in the frontal eye field of the monkey: evidence for independent eye and head control. J Neurophysiol. 2006;95:3528–3542. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
