Membrane-initiated estradiol signaling induces spinogenesis required for female sexual receptivity
- PMID: 22131419
- PMCID: PMC4709636
- DOI: 10.1523/JNEUROSCI.3030-11.2011
Membrane-initiated estradiol signaling induces spinogenesis required for female sexual receptivity
Abstract
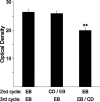
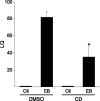
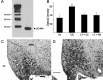
Estrogens have profound actions on the structure of the nervous system during development and in adulthood. One of the signature actions of estradiol is to alter the morphology of neural processes. In the hippocampus, estradiol modulates spines and cellular excitability that affect cognitive behaviors. In the hypothalamus, estradiol increases spine density in mediobasal hypothalamic nuclei that regulate reproduction. The hypothalamic arcuate nucleus (ARH), an important site for modulation of female sexual receptivity, has a sexual dimorphism in dendritic spine density that favors females. In the present study, we used both β-actin immunostaining and Golgi staining to visualize estradiol-induced changes in spine density in Long-Evans rats. Golgi impregnation was used to visualize spine shape, and then β-actin immunoreactivity was used as a semiquantitative measure of spine plasticity since actin forms the core of dendritic spines. At 4 h after estradiol treatment, both β-actin immunofluorescence and filopodial spines were increased (from 70.57 ± 1.09% to 78.01 ± 1.05%, p < 0.05). Disruption of estradiol-induced β-actin polymerization with cytochalasin D attenuated lordosis behavior, indicating the importance of estradiol-mediated spinogenesis for female sexual receptivity (81.43 ± 7.05 to 35.00 ± 11.76, p < 0.05). Deactivation of cofilin, an actin depolymerizing factor is required for spinogenesis. Membrane-initiated estradiol signaling involving the metabotropic glutamate receptor 1a was responsible for the phosphorylation and thereby deactivation of cofilin. These data demonstrate that estradiol-induced spinogenesis in the ARH is an important cellular mechanism for the regulation of female sexual behavior.
Figures

Similar articles
-
Temporal and concentration-dependent effects of oestradiol on neural pathways mediating sexual receptivity.J Neuroendocrinol. 2013 Nov;25(11):1012-23. doi: 10.1111/jne.12103. J Neuroendocrinol. 2013. PMID: 24028299 Free PMC article. Review.
-
Modulation of the arcuate nucleus-medial preoptic nucleus lordosis regulating circuit: a role for GABAB receptors.Horm Behav. 2013 Jun;64(1):136-43. doi: 10.1016/j.yhbeh.2013.06.001. Epub 2013 Jun 10. Horm Behav. 2013. PMID: 23756153 Free PMC article.
-
Protein kinase C signaling in the hypothalamic arcuate nucleus regulates sexual receptivity in female rats.Endocrinology. 2008 Dec;149(12):5934-42. doi: 10.1210/en.2008-0847. Epub 2008 Jul 24. Endocrinology. 2008. PMID: 18653714 Free PMC article.
-
17β-estradiol rapidly facilitates lordosis through G protein-coupled estrogen receptor 1 (GPER) via deactivation of medial preoptic nucleus μ-opioid receptors in estradiol primed female rats.Horm Behav. 2014 Sep;66(4):663-6. doi: 10.1016/j.yhbeh.2014.09.008. Epub 2014 Sep 22. Horm Behav. 2014. PMID: 25245158 Free PMC article.
-
Sexual dimorphism in estrogen-induced synaptogenesis in the adult hippocampus.Int J Dev Biol. 2013;57(5):351-6. doi: 10.1387/ijdb.120217gr. Int J Dev Biol. 2013. PMID: 23873366 Review.
Cited by
-
Membrane-initiated estradiol actions mediate structural plasticity and reproduction.Front Neuroendocrinol. 2012 Oct;33(4):331-41. doi: 10.1016/j.yfrne.2012.07.003. Epub 2012 Jul 22. Front Neuroendocrinol. 2012. PMID: 22828999 Free PMC article. Review.
-
Temporal and concentration-dependent effects of oestradiol on neural pathways mediating sexual receptivity.J Neuroendocrinol. 2013 Nov;25(11):1012-23. doi: 10.1111/jne.12103. J Neuroendocrinol. 2013. PMID: 24028299 Free PMC article. Review.
-
Membrane estrogen signaling in female reproduction and motivation.Front Endocrinol (Lausanne). 2022 Sep 29;13:1009379. doi: 10.3389/fendo.2022.1009379. eCollection 2022. Front Endocrinol (Lausanne). 2022. PMID: 36246891 Free PMC article. Review.
-
Opposite Effects of mGluR1a and mGluR5 Activation on Nucleus Accumbens Medium Spiny Neuron Dendritic Spine Density.PLoS One. 2016 Sep 12;11(9):e0162755. doi: 10.1371/journal.pone.0162755. eCollection 2016. PLoS One. 2016. PMID: 27618534 Free PMC article.
-
Gonadal Hormones Rapidly Enhance Spatial Memory and Increase Hippocampal Spine Density in Male Rats.Endocrinology. 2016 Apr;157(4):1357-62. doi: 10.1210/en.2015-1959. Epub 2016 Feb 4. Endocrinology. 2016. PMID: 26844375 Free PMC article.
References
-
- Bamburg JR. Proteins of the ADF/cofilin family: essential regulators of actin dynamics. Annu Rev Cell Dev Biol. 1999;15:185–230. - PubMed
-
- Csakvari E, Hoyk Z, Gyenes A, Garcia-Ovejero D, Garcia-Segura LM, Párducz A. Fluctuation of synapse density in the arcuate nucleus during the estrous cycle. Neuroscience. 2007;144:1288–1292. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
