Syntaxin1A lateral diffusion reveals transient and local SNARE interactions
- PMID: 22131420
- PMCID: PMC6623804
- DOI: 10.1523/JNEUROSCI.4065-11.2011
Syntaxin1A lateral diffusion reveals transient and local SNARE interactions
Abstract
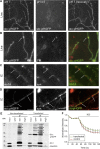
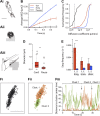
At the synapse, vesicles stably dock at the active zone. However, in cellular membranes, proteins undergo a diffusive motion. It is not known how the motion of membrane proteins involved in vesicle exocytosis is compatible with both vesicle docking and the dynamic remodeling of the plasma membrane imposed by cycles of exocytosis and endocytosis. To address this question, we studied the motion of the presynaptic membrane protein syntaxin1A at both the population and single-molecule levels in primary cultures of rat spinal cord neurons. Syntaxin1A was rapidly exchanged between synaptic and extrasynaptic regions. Changes in syntaxin1A mobility were associated with interactions related to the formation of the exocytotic complex. Finally, we propose a reaction-diffusion model reconciling the observed diffusive properties of syntaxin at the population level and at the molecular level. This work allows us to describe the diffusive behavior and kinetics of interactions between syntaxin1A and its partners that lead to its transient stabilization at the synapse.
Figures





Similar articles
-
In vivo single-molecule imaging of syntaxin1A reveals polyphosphoinositide- and activity-dependent trapping in presynaptic nanoclusters.Nat Commun. 2017 Jan 3;8:13660. doi: 10.1038/ncomms13660. Nat Commun. 2017. PMID: 28045048 Free PMC article.
-
A molecular toggle after exocytosis sequesters the presynaptic syntaxin1a molecules involved in prior vesicle fusion.Nat Commun. 2014 Dec 17;5:5774. doi: 10.1038/ncomms6774. Nat Commun. 2014. PMID: 25517944 Free PMC article.
-
SUMOylation of Syntaxin1A regulates presynaptic endocytosis.Sci Rep. 2015 Dec 4;5:17669. doi: 10.1038/srep17669. Sci Rep. 2015. PMID: 26635000 Free PMC article.
-
Need for speed: Super-resolving the dynamic nanoclustering of syntaxin-1 at exocytic fusion sites.Neuropharmacology. 2020 Jun 1;169:107554. doi: 10.1016/j.neuropharm.2019.02.036. Epub 2019 Feb 28. Neuropharmacology. 2020. PMID: 30826343 Review.
-
Distinct initial SNARE configurations underlying the diversity of exocytosis.Physiol Rev. 2012 Oct;92(4):1915-64. doi: 10.1152/physrev.00007.2012. Physiol Rev. 2012. PMID: 23073634 Review.
Cited by
-
Synaptic activity regulates the abundance and binding of complexin.Biophys J. 2015 Mar 24;108(6):1318-1329. doi: 10.1016/j.bpj.2014.12.057. Biophys J. 2015. PMID: 25809246 Free PMC article.
-
Two forms of asynchronous release with distinctive spatiotemporal dynamics in central synapses.Elife. 2023 May 11;12:e84041. doi: 10.7554/eLife.84041. Elife. 2023. PMID: 37166282 Free PMC article.
-
In vivo single-molecule imaging of syntaxin1A reveals polyphosphoinositide- and activity-dependent trapping in presynaptic nanoclusters.Nat Commun. 2017 Jan 3;8:13660. doi: 10.1038/ncomms13660. Nat Commun. 2017. PMID: 28045048 Free PMC article.
-
α-synuclein assemblies sequester neuronal α3-Na+/K+-ATPase and impair Na+ gradient.EMBO J. 2015 Oct 1;34(19):2408-23. doi: 10.15252/embj.201591397. Epub 2015 Aug 31. EMBO J. 2015. PMID: 26323479 Free PMC article.
-
Computational framework for single-cell spatiotemporal dynamics of optogenetic membrane recruitment.Cell Rep Methods. 2022 Jul 6;2(7):100245. doi: 10.1016/j.crmeth.2022.100245. eCollection 2022 Jul 18. Cell Rep Methods. 2022. PMID: 35880018 Free PMC article.
References
-
- Alcor D, Gouzer G, Triller A. Single-particle tracking methods for the study of membrane receptors dynamics. Eur J Neurosci. 2009;30:987–997. - PubMed
-
- Bannai H, Lévi S, Schweizer C, Inoue T, Launey T, Racine V, Sibarita JB, Mikoshiba K, Triller A. Activity-dependent tuning of inhibitory neurotransmission based on GABAAR diffusion dynamics. Neuron. 2009;62:670–682. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
