CCAAT/enhancer binding protein-δ expression by dendritic cells regulates CNS autoimmune inflammatory disease
- PMID: 22131422
- PMCID: PMC3334319
- DOI: 10.1523/JNEUROSCI.3449-11.2011
CCAAT/enhancer binding protein-δ expression by dendritic cells regulates CNS autoimmune inflammatory disease
Abstract
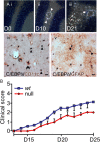
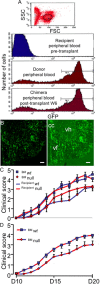
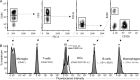
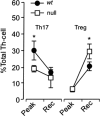
CCAAT enhancer binding protein-delta (C/EBPδ) is a transcription factor that regulates inflammatory processes mediating bystander neuronal injury and CNS autoimmune inflammatory disease. The mechanism of the involvement of C/EBPδ in these processes remains to be determined. Here, we examined the cellular source(s) and mechanisms by which C/EBPδ may be involved in an animal model of multiple sclerosis. Mice deficient in C/EBPδ expression exhibited less severe clinical disease than wild-type littermates in response to induction of experimental autoimmune encephalomyelitis (EAE) by vaccination with a myelin oligodendrocyte glycoprotein (MOG) fragment. This reduction in EAE severity was associated with a significant alteration in the complement of major CNS T-helper (Th) cell subtypes throughout disease, manifest as reduced ratios of Th17 cells to regulatory T-cells (Tregs). Studies in bone marrow chimeric mice indicated that C/EBPδ expression by peripherally derived immune cells mediates C/EBPδ involvement in EAE. Follow up in vitro and in vivo examination of dendritic cell (DC) mediated Th-cell development suggests that C/EBPδ suppresses DC expression of interleukin-10 (IL-10), favoring Th17 over Treg development. In vitro and in vivo blockade of IL-10 signaling attenuated the effect of reduced C/EBPδ expression by DCs on Th17:Treg ratios. These findings identify C/EBPδ as an important DC transcription factor in CNS autoimmune inflammatory disease by virtue of its capacity to alter the Th17:Treg balance in an IL-10 dependent fashion.
Figures


Similar articles
-
Involvement of regulatory T cells in the experimental autoimmune encephalomyelitis-preventive effect of dendritic cells expressing myelin oligodendrocyte glycoprotein plus TRAIL.J Immunol. 2007 Jan 15;178(2):918-25. doi: 10.4049/jimmunol.178.2.918. J Immunol. 2007. PMID: 17202353
-
IDO upregulates regulatory T cells via tryptophan catabolite and suppresses encephalitogenic T cell responses in experimental autoimmune encephalomyelitis.J Immunol. 2010 Nov 15;185(10):5953-61. doi: 10.4049/jimmunol.1001628. Epub 2010 Oct 13. J Immunol. 2010. PMID: 20944000 Free PMC article.
-
Chemokine CCL17 is expressed by dendritic cells in the CNS during experimental autoimmune encephalomyelitis and promotes pathogenesis of disease.Brain Behav Immun. 2017 Nov;66:382-393. doi: 10.1016/j.bbi.2017.06.010. Epub 2017 Jun 19. Brain Behav Immun. 2017. PMID: 28642092
-
Functional and pathogenic differences of Th1 and Th17 cells in experimental autoimmune encephalomyelitis.PLoS One. 2010 Nov 29;5(11):e15531. doi: 10.1371/journal.pone.0015531. PLoS One. 2010. PMID: 21209700 Free PMC article.
-
Role of Th17 cells in the pathogenesis of CNS inflammatory demyelination.J Neurol Sci. 2013 Oct 15;333(1-2):76-87. doi: 10.1016/j.jns.2013.03.002. Epub 2013 Apr 8. J Neurol Sci. 2013. PMID: 23578791 Free PMC article. Review.
Cited by
-
Identification and Characterisation of cis-Regulatory Elements Upstream of the Human Receptor Tyrosine Kinase Gene MERTK.Brain Plast. 2021 Aug 23;7(1):3-16. doi: 10.3233/BPL-200102. eCollection 2021. Brain Plast. 2021. PMID: 34631417 Free PMC article.
-
FBXW7α attenuates inflammatory signalling by downregulating C/EBPδ and its target gene Tlr4.Nat Commun. 2013;4:1662. doi: 10.1038/ncomms2677. Nat Commun. 2013. PMID: 23575666 Free PMC article.
-
Dendritic cells and multiple sclerosis: disease, tolerance and therapy.Int J Mol Sci. 2012 Dec 27;14(1):547-62. doi: 10.3390/ijms14010547. Int J Mol Sci. 2012. PMID: 23271370 Free PMC article. Review.
-
In-vitro characterization of novel and functional regulatory SNPs in the promoter region of IL2 and IL2R alpha in a Gabonese population.BMC Med Genet. 2012 Dec 7;13:117. doi: 10.1186/1471-2350-13-117. BMC Med Genet. 2012. PMID: 23217119 Free PMC article.
-
Enhanced killing of chordoma cells by antibody-dependent cell-mediated cytotoxicity employing the novel anti-PD-L1 antibody avelumab.Oncotarget. 2016 Jun 7;7(23):33498-511. doi: 10.18632/oncotarget.9256. Oncotarget. 2016. PMID: 27172898 Free PMC article.
References
-
- Alam T, An MR, Papaconstantinou J. Differential expression of three C/EBP isoforms in multiple tissues during the acute phase response. J Biol Chem. 1992;267:5021–5024. - PubMed
-
- Bailey SL, Schreiner B, McMahon EJ, Miller SD. CNS myeloid DCs presenting endogenous myelin peptides ‘preferentially’ polarize CD4(+) T(H)-17 cells in relapsing EAE. Nat Immunol. 2007;8:172–180. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
