Alterations in intrinsic membrane properties and the axon initial segment in a mouse model of Angelman syndrome
- PMID: 22131424
- PMCID: PMC3483031
- DOI: 10.1523/JNEUROSCI.4162-11.2011
Alterations in intrinsic membrane properties and the axon initial segment in a mouse model of Angelman syndrome
Abstract
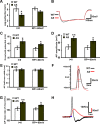
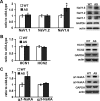
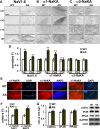
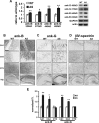
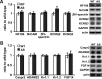
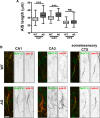
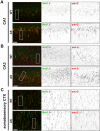
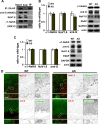
The axon initial segment (AIS) is the site of action potential initiation in neurons. Recent studies have demonstrated activity-dependent regulation of the AIS, including homeostatic changes in AIS length, membrane excitability, and the localization of voltage-gated Na(+) channels. The neurodevelopmental disorder Angelman syndrome (AS) is usually caused by the deletion of small portions of the maternal copy of chromosome 15, which includes the UBE3A gene. A mouse model of AS has been generated and these mice exhibit multiple neurological abnormalities similar to those observed in humans. We examined intrinsic properties of pyramidal neurons in hippocampal area CA1 from AS model mice and observed alterations in resting membrane potential, threshold potential, and action potential amplitude. The altered intrinsic properties in the AS mice were correlated with significant increases in the expression of the α1 subunit of Na/K-ATPase (α1-NaKA), the Na(+) channel NaV1.6, and the AIS anchoring protein ankyrin-G, as well as an increase in length of the AIS. These findings are the first evidence for pathology of intrinsic membrane properties and AIS-specific changes in AS, a neurodevelopmental disorder associated with autism.
Figures

Similar articles
-
Chronic α1-Na/K-ATPase inhibition reverses the elongation of the axon initial segment of the hippocampal CA1 pyramidal neurons in Angelman syndrome model mice.Neuropsychopharmacology. 2021 Feb;46(3):654-664. doi: 10.1038/s41386-020-00907-1. Epub 2020 Nov 19. Neuropsychopharmacology. 2021. PMID: 33214655 Free PMC article.
-
Genetic reduction of the α1 subunit of Na/K-ATPase corrects multiple hippocampal phenotypes in Angelman syndrome.Cell Rep. 2013 Aug 15;4(3):405-12. doi: 10.1016/j.celrep.2013.07.005. Epub 2013 Aug 1. Cell Rep. 2013. PMID: 23911285 Free PMC article.
-
Role of axonal NaV1.6 sodium channels in action potential initiation of CA1 pyramidal neurons.J Neurophysiol. 2008 Oct;100(4):2361-80. doi: 10.1152/jn.90332.2008. Epub 2008 Jul 23. J Neurophysiol. 2008. PMID: 18650312
-
Neuronal signaling in central nervous system.Sheng Li Xue Bao. 2011 Feb 25;63(1):1-8. Sheng Li Xue Bao. 2011. PMID: 21340428 Review.
-
Signal processing in the axon initial segment.Neuron. 2012 Jan 26;73(2):235-47. doi: 10.1016/j.neuron.2012.01.007. Neuron. 2012. PMID: 22284179 Review.
Cited by
-
IPSC Models of Chromosome 15Q Imprinting Disorders: From Disease Modeling to Therapeutic Strategies.Adv Neurobiol. 2020;25:55-77. doi: 10.1007/978-3-030-45493-7_3. Adv Neurobiol. 2020. PMID: 32578144 Free PMC article.
-
The Changes of Intrinsic Excitability of Pyramidal Neurons in Anterior Cingulate Cortex in Neuropathic Pain.Front Cell Neurosci. 2018 Nov 21;12:436. doi: 10.3389/fncel.2018.00436. eCollection 2018. Front Cell Neurosci. 2018. PMID: 30519160 Free PMC article.
-
Adult Ube3a Gene Reinstatement Restores the Electrophysiological Deficits of Prefrontal Cortex Layer 5 Neurons in a Mouse Model of Angelman Syndrome.J Neurosci. 2018 Sep 12;38(37):8011-8030. doi: 10.1523/JNEUROSCI.0083-18.2018. Epub 2018 Aug 6. J Neurosci. 2018. PMID: 30082419 Free PMC article.
-
Na,K-ATPase β1-subunit is a target of sonic hedgehog signaling and enhances medulloblastoma tumorigenicity.Mol Cancer. 2015 Aug 19;14:159. doi: 10.1186/s12943-015-0430-1. Mol Cancer. 2015. PMID: 26286140 Free PMC article.
-
Assembly and Function of the Juxtaparanodal Kv1 Complex in Health and Disease.Life (Basel). 2020 Dec 24;11(1):8. doi: 10.3390/life11010008. Life (Basel). 2020. PMID: 33374190 Free PMC article. Review.
References
-
- Albrecht U, Sutcliffe JS, Cattanach BM, Beechey CV, Armstrong D, Eichele G, Beaudet AL. Imprinted expression of the murine Angelman syndrome gene, Ube3a, in hippocampal and Purkinje neurons. Nat Genet. 1997;17:75–78. - PubMed
-
- Al-Mosalem OA, El-Ansary A, Attas O, Al-Ayadhi L. Metabolic biomarkers related to energy metabolism in Saudi autistic children. Clin Biochem. 2009;42:949–957. - PubMed
-
- Blaustein MP, Lederer WJ. Sodium/calcium exchange: its physiological implications. Physiol Rev. 1999;79:763–854. - PubMed
-
- Boda B, Dubos A, Muller D. Signaling mechanisms regulating synapse formation and function in mental retardation. Curr Opin Neurobiol. 2010;20:519–527. - PubMed
-
- Deng V, Matagne V, Banine F, Frerking M, Ohliger P, Budden S, Pevsner J, Dissen GA, Sherman LS, Ojeda SR. FXYD1 is an MeCP2 target gene overexpressed in the brains of Rett syndrome patients and Mecp2-null mice. Hum Mol Genet. 2007;16:640–650. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous
