Opioid-sensitive GABA inputs from rostromedial tegmental nucleus synapse onto midbrain dopamine neurons
- PMID: 22131433
- PMCID: PMC3617570
- DOI: 10.1523/JNEUROSCI.4570-11.2011
Opioid-sensitive GABA inputs from rostromedial tegmental nucleus synapse onto midbrain dopamine neurons
Abstract
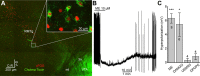
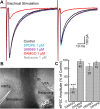
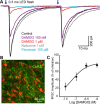
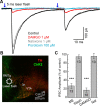
Opioids increase dopamine release in the brain through inhibition of GABA-A IPSCs onto dopamine cells. Immunolabeling indicates that GABA neurons in the rostromedial tegmental nucleus (RMTg), also known as the tail of the ventral tegmental area, send a dense projection to midbrain dopamine neurons stain for μ-opioid receptors. There is however, little functional evidence that these neurons play a role in the opioid-dependent increase in dopamine neuron activity. The present study used retrograde tracers injected into the ventral tegmental area and substantia nigra (VTA/SN) to identify RMTg neurons that project to the VTA/SN. Whole-cell current-clamp and cell-attached recordings from labeled RMTg neurons were performed in sagittal slices from rat. The rhythmic spontaneous firing rate of RMTg neurons was decreased and the membrane potential was hyperpolarized in response to application of μ-opioid agonist DAMGO. Agonists that act at κ- and δ-opioid receptors (U69593 and DPDPE) failed to hyperpolarize RMTg neurons. Whole-cell recordings made in dopamine neurons revealed rhythmic, large amplitude spontaneous IPSCs that had a similar frequency, pattern and opioid sensitivity to the firing of RMTg neurons. In addition, electrical and channelrhodopsin-2 stimulation within the RMTg evoked GABA-A IPSCs in dopamine neurons that were inhibited by μ-opioid agonists DAMGO, but not κ- and δ-opioid agonists. Thus, this study demonstrates functional connection from the RMTg to the VTA/SN mediated by a dense, opioid-sensitive GABA innervation, and that the RMTg is a key structure in the μ-opioid receptor-dependent regulation of dopamine neurons.
Figures

Similar articles
-
Ethanol dually modulates GABAergic synaptic transmission onto dopaminergic neurons in ventral tegmental area: role of mu-opioid receptors.Neuroscience. 2008 Apr 22;153(1):240-8. doi: 10.1016/j.neuroscience.2008.01.040. Epub 2008 Feb 6. Neuroscience. 2008. PMID: 18343590 Free PMC article.
-
Inhibitory inputs from rostromedial tegmental neurons regulate spontaneous activity of midbrain dopamine cells and their responses to drugs of abuse.Neuropsychopharmacology. 2012 Apr;37(5):1164-76. doi: 10.1038/npp.2011.302. Epub 2011 Dec 14. Neuropsychopharmacology. 2012. PMID: 22169942 Free PMC article.
-
The rostromedial tegmental nucleus: a key modulator of pain and opioid analgesia.Pain. 2019 Nov;160(11):2524-2534. doi: 10.1097/j.pain.0000000000001647. Pain. 2019. PMID: 31246732 Free PMC article.
-
Progress in opioid reward research: From a canonical two-neuron hypothesis to two neural circuits.Pharmacol Biochem Behav. 2021 Jan;200:173072. doi: 10.1016/j.pbb.2020.173072. Epub 2020 Nov 20. Pharmacol Biochem Behav. 2021. PMID: 33227308 Free PMC article. Review.
-
Opioid-induced rewards, locomotion, and dopamine activation: A proposed model for control by mesopontine and rostromedial tegmental neurons.Neurosci Biobehav Rev. 2017 Dec;83:72-82. doi: 10.1016/j.neubiorev.2017.09.022. Epub 2017 Sep 23. Neurosci Biobehav Rev. 2017. PMID: 28951251 Free PMC article. Review.
Cited by
-
Salsolinol modulation of dopamine neurons.Front Behav Neurosci. 2013 May 24;7:52. doi: 10.3389/fnbeh.2013.00052. eCollection 2013. Front Behav Neurosci. 2013. PMID: 23745110 Free PMC article.
-
Identification of rat ventral tegmental area GABAergic neurons.PLoS One. 2012;7(7):e42365. doi: 10.1371/journal.pone.0042365. Epub 2012 Jul 31. PLoS One. 2012. PMID: 22860119 Free PMC article.
-
Optogenetic dissection of neural circuits underlying emotional valence and motivated behaviors.Brain Res. 2013 May 20;1511:73-92. doi: 10.1016/j.brainres.2012.11.001. Epub 2012 Nov 8. Brain Res. 2013. PMID: 23142759 Free PMC article. Review.
-
The role of enkephalinergic systems in substance use disorders.Front Syst Neurosci. 2022 Aug 5;16:932546. doi: 10.3389/fnsys.2022.932546. eCollection 2022. Front Syst Neurosci. 2022. PMID: 35993087 Free PMC article. Review.
-
Poststress block of kappa opioid receptors rescues long-term potentiation of inhibitory synapses and prevents reinstatement of cocaine seeking.Biol Psychiatry. 2014 Nov 15;76(10):785-93. doi: 10.1016/j.biopsych.2014.04.019. Epub 2014 May 21. Biol Psychiatry. 2014. PMID: 24957331 Free PMC article.
References
-
- Blackmer T, Larsen EC, Bartleson C, Kowalchyk JA, Yoon EJ, Preininger AM, Alford S, Hamm HE, Martin TF. G protein betagamma directly regulates SNARE protein fusion machinery for secretory granule exocytosis. Nat Neurosci. 2005;8:421–425. - PubMed
-
- Bonci A, Williams JT. A common mechanism mediates long-term changes in synaptic transmission after chronic cocaine and morphine. Neuron. 1996;16:631–639. - PubMed
-
- Cameron DL, Wessendorf MW, Williams JT. A subset of ventral tegmental area neurons is inhibited by dopamine, 5-hydroxytryptamine and opioids. Neuroscience. 1997;77:155–166. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
