Congenital CNS hypomyelination in the Fig4 null mouse is rescued by neuronal expression of the PI(3,5)P(2) phosphatase Fig4
- PMID: 22131434
- PMCID: PMC3711465
- DOI: 10.1523/JNEUROSCI.1482-11.2011
Congenital CNS hypomyelination in the Fig4 null mouse is rescued by neuronal expression of the PI(3,5)P(2) phosphatase Fig4
Abstract
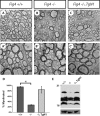
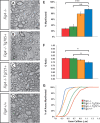
The plt (pale tremor) mouse carries a null mutation in the Fig4(Sac3) gene that results in tremor, hypopigmentation, spongiform degeneration of the brain, and juvenile lethality. FIG4 is a ubiquitously expressed phosphatidylinositol 3,5-bisphosphate phosphatase that regulates intracellular vesicle trafficking along the endosomal-lysosomal pathway. In humans, the missense mutation FIG4(I41T) combined with a FIG4 null allele causes Charcot-Marie-Tooth 4J disease, a severe form of peripheral neuropathy. Here we show that Fig4 null mice exhibit a dramatic reduction of myelin in the brain and spinal cord. In the optic nerve, smaller-caliber axons lack myelin sheaths entirely, whereas many large- and intermediate-caliber axons are myelinated but show structural defects at nodes of Ranvier, leading to delayed propagation of action potentials. In the Fig4 null brain and optic nerve, oligodendrocyte (OL) progenitor cells are present at normal abundance and distribution, but the number of myelinating OLs is greatly compromised. The total number of axons in the Fig4 null optic nerve is not reduced. Developmental studies reveal incomplete myelination rather than elevated cell death in the OL linage. Strikingly, there is rescue of CNS myelination and tremor in transgenic mice with neuron-specific expression of Fig4, demonstrating a non-cell-autonomous function of Fig4 in OL maturation and myelin development. In transgenic mice with global overexpression of the human pathogenic FIG4 variant I41T, there is rescue of the myelination defect, suggesting that the CNS of CMT4J patients may be protected from myelin deficiency by expression of the FIG4(I41T) mutant protein.
Figures






Similar articles
-
Protective role of the lipid phosphatase Fig4 in the adult nervous system.Hum Mol Genet. 2018 Jul 15;27(14):2443-2453. doi: 10.1093/hmg/ddy145. Hum Mol Genet. 2018. PMID: 29688489 Free PMC article.
-
Genetic interaction between MTMR2 and FIG4 phospholipid phosphatases involved in Charcot-Marie-Tooth neuropathies.PLoS Genet. 2011 Oct;7(10):e1002319. doi: 10.1371/journal.pgen.1002319. Epub 2011 Oct 20. PLoS Genet. 2011. PMID: 22028665 Free PMC article.
-
Pathogenic mechanism of the FIG4 mutation responsible for Charcot-Marie-Tooth disease CMT4J.PLoS Genet. 2011 Jun;7(6):e1002104. doi: 10.1371/journal.pgen.1002104. Epub 2011 Jun 2. PLoS Genet. 2011. PMID: 21655088 Free PMC article.
-
Fig4 deficiency: a newly emerged lysosomal storage disorder?Prog Neurobiol. 2013 Feb-Mar;101-102:35-45. doi: 10.1016/j.pneurobio.2012.11.001. Epub 2012 Nov 16. Prog Neurobiol. 2013. PMID: 23165282 Free PMC article. Review.
-
FIG4-Related Parkinsonism and the Particularities of the I41T Mutation: A Review of the Literature.Genes (Basel). 2024 Oct 21;15(10):1344. doi: 10.3390/genes15101344. Genes (Basel). 2024. PMID: 39457468 Free PMC article. Review.
Cited by
-
Neuronal expression of Fig4 is both necessary and sufficient to prevent spongiform neurodegeneration.Hum Mol Genet. 2012 Aug 15;21(16):3525-34. doi: 10.1093/hmg/dds179. Epub 2012 May 11. Hum Mol Genet. 2012. PMID: 22581779 Free PMC article.
-
Rescue of neurodegeneration in the Fig4 null mouse by a catalytically inactive FIG4 transgene.Hum Mol Genet. 2016 Jan 15;25(2):340-7. doi: 10.1093/hmg/ddv480. Epub 2015 Nov 24. Hum Mol Genet. 2016. PMID: 26604144 Free PMC article.
-
Transient auditory nerve demyelination as a new mechanism for hidden hearing loss.Nat Commun. 2017 Feb 17;8:14487. doi: 10.1038/ncomms14487. Nat Commun. 2017. PMID: 28211470 Free PMC article.
-
Npc1 acting in neurons and glia is essential for the formation and maintenance of CNS myelin.PLoS Genet. 2013 Apr;9(4):e1003462. doi: 10.1371/journal.pgen.1003462. Epub 2013 Apr 11. PLoS Genet. 2013. PMID: 23593041 Free PMC article.
-
Pathophysiological Heterogeneity of the BBSOA Neurodevelopmental Syndrome.Cells. 2022 Apr 8;11(8):1260. doi: 10.3390/cells11081260. Cells. 2022. PMID: 35455940 Free PMC article. Review.
References
-
- Barres BA, Raff MC. Control of oligodendrocyte number in the developing rat optic nerve. Neuron. 1994;12:935–942. - PubMed
-
- Barres BA, Hart IK, Coles HS, Burne JF, Voyvodic JT, Richardson WD, Raff MC. Cell death and control of cell survival in the oligodendrocyte lineage. Cell. 1992;70:31–46. - PubMed
-
- Botelho RJ. Changing phosphoinositides “on the fly”: how trafficking vesicles avoid an identity crisis. Bioessays. 2009;31:1127–1136. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous
