Colocalization of serum amyloid a with microtubules in human coronary artery endothelial cells
- PMID: 22131810
- PMCID: PMC3205747
- DOI: 10.1155/2011/528276
Colocalization of serum amyloid a with microtubules in human coronary artery endothelial cells
Abstract
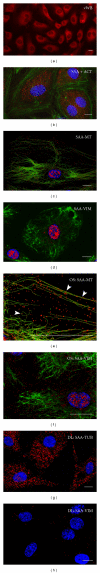
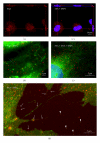
Serum amyloid A (SAA) acts as a major acute phase protein and represents a sensitive and accurate marker of inflammation. Besides its hepatic origin, as the main source of serum SAA, this protein is also produced extrahepatically. The mRNA levels of SAA become significantly elevated following proinflammatory stimuli, as well as, are induced through their own positive feedback in human primary coronary artery endothelial cells. However, the intracellular functions of SAA are so far unknown. Colocalization of SAA with cytoskeletal filaments has previously been proposed, so we analyzed the colocalization of SAA with all three cytoskeletal elements: actin filaments, vimentin filaments, and microtubules. Immunofluorescent double-labeling analyses confirmed by PLA method revealed a strict colocalization of SAA with microtubules and a very infrequent attachment to vimentin while the distribution of actin filaments appeared clearly separated from SAA staining. Also, no significant colocalization was found between SAA and endomembranes labeled with the fluorescent lipid stain DiO₆. However, SAA appears to be located also unbound in the cytosol, as well as inside the nucleus and within nanotubes extending from the cells or bridging neighboring cells. These different locations of SAA in endothelial cells strongly indicate multiple potential functions of this protein.
Figures

Similar articles
-
Serum amyloid A induces endothelial dysfunction in porcine coronary arteries and human coronary artery endothelial cells.Am J Physiol Heart Circ Physiol. 2008 Dec;295(6):H2399-408. doi: 10.1152/ajpheart.00238.2008. Epub 2008 Oct 17. Am J Physiol Heart Circ Physiol. 2008. PMID: 18931033 Free PMC article.
-
[Endothelial cell cytoskeleton reorganization during functional monolayer formation in vitro].Tsitologiia. 2014;56(1):36-47. Tsitologiia. 2014. PMID: 25509142 Russian.
-
Reorientation dynamics and structural interdependencies of actin, microtubules and intermediate filaments upon cyclic stretch application.Cytoskeleton (Hoboken). 2018 Sep;75(9):385-394. doi: 10.1002/cm.21470. Epub 2018 Oct 12. Cytoskeleton (Hoboken). 2018. PMID: 30176121
-
A role for acute-phase serum amyloid A and high-density lipoprotein in oxidative stress, endothelial dysfunction and atherosclerosis.Redox Rep. 2009;14(5):187-96. doi: 10.1179/135100009X12525712409490. Redox Rep. 2009. PMID: 19843373 Review.
-
Joining actions: crosstalk between intermediate filaments and actin orchestrates cellular physical dynamics and signaling.Sci China Life Sci. 2019 Oct;62(10):1368-1374. doi: 10.1007/s11427-018-9488-1. Epub 2019 May 14. Sci China Life Sci. 2019. PMID: 31098891 Review.
Cited by
-
C-reactive protein and serum amyloid a overexpression in lung tissues of chronic obstructive pulmonary disease patients: a case-control study.Int J Med Sci. 2013 Jun 8;10(8):938-47. doi: 10.7150/ijms.6152. Print 2013. Int J Med Sci. 2013. PMID: 23801879 Free PMC article.
-
No effects without causes: the Iron Dysregulation and Dormant Microbes hypothesis for chronic, inflammatory diseases.Biol Rev Camb Philos Soc. 2018 Aug;93(3):1518-1557. doi: 10.1111/brv.12407. Epub 2018 Mar 25. Biol Rev Camb Philos Soc. 2018. PMID: 29575574 Free PMC article. Review.
-
NFκB Inhibition Mitigates Serum Amyloid A-Induced Pro-Atherogenic Responses in Endothelial Cells and Leukocyte Adhesion and Adverse Changes to Endothelium Function in Isolated Aorta.Int J Mol Sci. 2018 Dec 28;20(1):105. doi: 10.3390/ijms20010105. Int J Mol Sci. 2018. PMID: 30597899 Free PMC article.
-
Serum amyloid A in the placenta and its role in trophoblast invasion.PLoS One. 2014 Mar 10;9(3):e90881. doi: 10.1371/journal.pone.0090881. eCollection 2014. PLoS One. 2014. PMID: 24614130 Free PMC article.
-
Editorial: Molecular profiles of tunneling nanotubes (TNTs) in human diseases-from 2D cultures to complex tissue.Front Cell Dev Biol. 2024 Aug 14;12:1461453. doi: 10.3389/fcell.2024.1461453. eCollection 2024. Front Cell Dev Biol. 2024. PMID: 39211391 Free PMC article. No abstract available.
References
-
- Gabay C, Kushner I. Acute-phase proteins and other systemic responses to inflammation. The New England Journal of Medicine. 1999;340(6):448–454. - PubMed
-
- Malle E, De Beer FC. Human serum amyloid A (SAA) protein: a prominent acute-phase reactant for clinical practice. European Journal of Clinical Investigation. 1996;26(6):427–435. - PubMed
-
- Urieli-Shoval S, Cohen P, Eisenberg S, Matzner Y. Widespread expression of serum amyloida A in histologically normal human tissues: predominant localization to the epithelium. Journal of Histochemistry and Cytochemistry. 1998;46(12):1377–1384. - PubMed
-
- Uhlar CM, Burgess CJ, Sharp PM, Whitehead AS. Evolution of the serum amyloid A (SAA) protein superfamily. Genomics. 1994;19(2):228–235. - PubMed
-
- Santiago P, Roig-López JL, Santiago C, García-Arrarás JE. Serum amyloid a protein in an echinoderm: its primary structure and expression during intestinal regeneration in the sea cucumber Holothuria glaberrima. Journal of Experimental Zoology. 2000;288(4):335–344. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

