Alpha 1,3-galactosyltransferase deficiency in pigs increases sialyltransferase activities that potentially raise non-gal xenoantigenicity
- PMID: 22131812
- PMCID: PMC3205825
- DOI: 10.1155/2011/560850
Alpha 1,3-galactosyltransferase deficiency in pigs increases sialyltransferase activities that potentially raise non-gal xenoantigenicity
Abstract
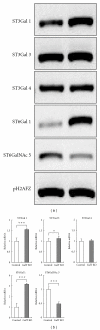
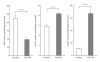
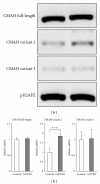
We examined whether deficiency of the GGTA1 gene in pigs altered the expression of several glycosyltransferase genes. Real-time RT-PCR and glycosyltransferase activity showed that 2 sialyltransferases [α2,3-sialyltransferase (α2,3ST) and α2,6-sialyltransferase (α2,6ST)] in the heterozygote GalT KO liver have higher expression levels and activities compared to controls. Enzyme-linked lectin assays indicated that there were also more sialic acid-containing glycoconjugate epitopes in GalT KO livers than in controls. The elevated level of sialic-acid-containing glycoconjugate epitopes was due to the low level of α-Gal in heterozygote GalT KO livers. Furthermore, proteomics analysis showed that heterozygote GalT KO pigs had a higher expression of NAD+-isocitrate dehydrogenase (IDH), which is related to the CMP-N-acetylneuraminic acid hydroxylase (CMAH) enzyme reaction. These findings suggest the deficiency of GGTA1 gene in pigs results in increased production of N-glycolylneuraminic acid (Neu5Gc) due to an increase of α2,6-sialyltransferase and a CMAH cofactor, NAD+-IDH. This indicates that Neu5Gc may be a critical xenoantigen. The deletion of the CMAH gene in the GalT KO background is expected to further prolong xenograft survival.
Figures

Similar articles
-
Generation of cattle knockout for galactose-α1,3-galactose and N-glycolylneuraminic acid antigens.Xenotransplantation. 2019 Sep;26(5):e12524. doi: 10.1111/xen.12524. Epub 2019 May 22. Xenotransplantation. 2019. PMID: 31115108 Free PMC article.
-
Erythrocytes from GGTA1/CMAH knockout pigs: implications for xenotransfusion and testing in non-human primates.Xenotransplantation. 2014 Jul-Aug;21(4):376-84. doi: 10.1111/xen.12106. Epub 2014 Jul 2. Xenotransplantation. 2014. PMID: 24986655 Free PMC article.
-
Reduction of the major swine xenoantigen, the alpha-galactosyl epitope by transfection of the alpha2,3-sialyltransferase gene.J Biol Chem. 1998 Jun 26;273(26):16421-5. doi: 10.1074/jbc.273.26.16421. J Biol Chem. 1998. PMID: 9632707
-
Immune responses to alpha1,3 galactosyltransferase knockout pigs.Curr Opin Organ Transplant. 2009 Apr;14(2):154-60. doi: 10.1097/MOT.0b013e328329250d. Curr Opin Organ Transplant. 2009. PMID: 19300259 Review.
-
N-Glycolylneuraminic Acid (Neu5Gc) Null Large Animals by Targeting the CMP-Neu5Gc Hydroxylase (CMAH).Front Immunol. 2019 Oct 15;10:2396. doi: 10.3389/fimmu.2019.02396. eCollection 2019. Front Immunol. 2019. PMID: 31681287 Free PMC article. Review.
Cited by
-
Inclusion of homologous DNA in nuclease-mediated gene targeting facilitates a higher incidence of bi-allelically modified cells.Xenotransplantation. 2015 Sep-Oct;22(5):379-90. doi: 10.1111/xen.12194. Xenotransplantation. 2015. PMID: 26381494 Free PMC article.
-
Immunological challenges and therapies in xenotransplantation.Cold Spring Harb Perspect Med. 2014 Apr 1;4(4):a015578. doi: 10.1101/cshperspect.a015578. Cold Spring Harb Perspect Med. 2014. PMID: 24616201 Free PMC article. Review.
-
Differential Role of B Cells and IL-17 Versus IFN-γ During Early and Late Rejection of Pig Islet Xenografts in Mice.Transplantation. 2017 Aug;101(8):1801-1810. doi: 10.1097/TP.0000000000001489. Transplantation. 2017. PMID: 27893617 Free PMC article.
-
Cutting edge of immune response and immunosuppressants in allogeneic and xenogeneic islet transplantation.Front Immunol. 2024 Sep 13;15:1455691. doi: 10.3389/fimmu.2024.1455691. eCollection 2024. Front Immunol. 2024. PMID: 39346923 Free PMC article. Review.
-
The Role of NK Cells in Pig-to-Human Xenotransplantation.J Immunol Res. 2017;2017:4627384. doi: 10.1155/2017/4627384. Epub 2017 Dec 19. J Immunol Res. 2017. PMID: 29410970 Free PMC article. Review.
References
-
- Sandrin MS, McKenzie IF. Gal α(1,3)Gal, the major xenoantigen(s) recognised in pigs by human natural antibodies. Immunological Reviews. 1994;(141):169–190. - PubMed
-
- Galili U, Shohet SB, Kobrin E, Stults CL, Macher BA. Man, apes, and Old World monkeys differ from other mammals in the expression of alpha-galactosyl epitopes on nucleated cells. Journal of Biological Chemistry. 1988;263(33):17755–17762. - PubMed
-
- Dai Y, Vaught TD, Boone J, et al. Targeted disruption of the α1,3-galactosyltransferase gene in cloned pigs. Nature Biotechnology. 2002;20(3):251–255. - PubMed
-
- Lai L, Kolber-Simonds D, Park KW, et al. Production of α-1,3-galactosyltransferase knockout pigs by nuclear transfer cloning. Science. 2002;295(5557):1089–1092. - PubMed
-
- Kuwaki K, Tseng YL, Dor FJ, et al. Heart transplantation in baboons using α1,3-galactosyltransferase gene-knockout pigs as donors: initial experience. Nature Medicine. 2005;11(1):29–31. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
