Coordinated regulation of niche and stem cell precursors by hormonal signaling
- PMID: 22131903
- PMCID: PMC3222635
- DOI: 10.1371/journal.pbio.1001202
Coordinated regulation of niche and stem cell precursors by hormonal signaling
Abstract
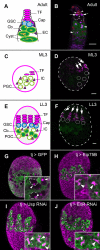
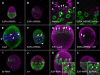
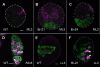
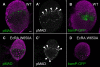
Stem cells and their niches constitute units that act cooperatively to achieve adult body homeostasis. How such units form and whether stem cell and niche precursors might be coordinated already during organogenesis are unknown. In fruit flies, primordial germ cells (PGCs), the precursors of germ line stem cells (GSCs), and somatic niche precursors develop within the larval ovary. Together they form the 16-20 GSC units of the adult ovary. We show that ecdysone receptors are required to coordinate the development of niche and GSC precursors. At early third instar, ecdysone receptors repress precocious differentiation of both niches and PGCs. Early repression is required for correct morphogenesis of the ovary and for protecting future GSCs from differentiation. At mid-third instar, ecdysone signaling is required for niche formation. Finally, and concurrent with the initiation of wandering behavior, ecdysone signaling initiates PGC differentiation by allowing the expression of the differentiation gene bag of marbles in PGCs that are not protected by the newly formed niches. All the ovarian functions of ecdysone receptors are mediated through early repression, and late activation, of the ecdysone target gene broad. These results show that, similar to mammals, a brain-gland-gonad axis controls the initiation of oogenesis in insects. They further exemplify how a physiological cue coordinates the formation of a stem cell unit within an organ: it is required for niche establishment and to ensure that precursor cells to adult stem cells remain undifferentiated until the niches can accommodate them. Similar principles might govern the formation of additional stem cell units during organogenesis.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


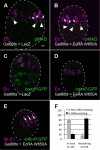
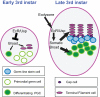
Similar articles
-
Insulin and Target of rapamycin signaling orchestrate the development of ovarian niche-stem cell units in Drosophila.Development. 2013 Oct;140(20):4145-54. doi: 10.1242/dev.093773. Epub 2013 Sep 11. Development. 2013. PMID: 24026119
-
Activin signaling balances proliferation and differentiation of ovarian niche precursors and enables adjustment of niche numbers.Development. 2015 Mar 1;142(5):883-92. doi: 10.1242/dev.113902. Epub 2015 Jan 29. Development. 2015. PMID: 25633355
-
MicroRNA-dependent roles of Drosha and Pasha in the Drosophila larval ovary morphogenesis.Dev Biol. 2016 Aug 15;416(2):312-23. doi: 10.1016/j.ydbio.2016.06.026. Epub 2016 Jun 20. Dev Biol. 2016. PMID: 27339292
-
Molecular control of the female germline stem cell niche size in Drosophila.Cell Mol Life Sci. 2019 Nov;76(21):4309-4317. doi: 10.1007/s00018-019-03223-0. Epub 2019 Jul 12. Cell Mol Life Sci. 2019. PMID: 31300869 Free PMC article. Review.
-
Organizing stem cell units in the Drosophila ovary.Curr Opin Genet Dev. 2015 Jun;32:31-6. doi: 10.1016/j.gde.2015.01.005. Epub 2015 Feb 19. Curr Opin Genet Dev. 2015. PMID: 25703842 Review.
Cited by
-
The Osa-Containing SWI/SNF Chromatin-Remodeling Complex Is Required in the Germline Differentiation Niche for Germline Stem Cell Progeny Differentiation.Genes (Basel). 2021 Mar 4;12(3):363. doi: 10.3390/genes12030363. Genes (Basel). 2021. PMID: 33806269 Free PMC article.
-
Cyromazine affects the ovarian germ cells of Drosophila via the ecdysone signaling pathway.Front Physiol. 2022 Sep 29;13:992306. doi: 10.3389/fphys.2022.992306. eCollection 2022. Front Physiol. 2022. PMID: 36246127 Free PMC article.
-
Steroid signaling controls sex-specific development in an invertebrate.bioRxiv [Preprint]. 2024 Jun 26:2023.12.22.573099. doi: 10.1101/2023.12.22.573099. bioRxiv. 2024. PMID: 38187640 Free PMC article. Preprint.
-
Insulin signalling underlies both plasticity and divergence of a reproductive trait in Drosophila.Proc Biol Sci. 2014 Feb 5;281(1779):20132673. doi: 10.1098/rspb.2013.2673. Print 2014 Mar 22. Proc Biol Sci. 2014. PMID: 24500165 Free PMC article.
-
Hardwiring Stem Cell Communication through Tissue Structure.Cell. 2016 Mar 10;164(6):1212-1225. doi: 10.1016/j.cell.2016.02.041. Cell. 2016. PMID: 26967287 Free PMC article. Review.
References
-
- Fuller M. T, Spradling A. C. Male and female Drosophila germline stem cells: two versions of immortality. Science. 2007;316:402–404. - PubMed
-
- Kirilly D, Xie T. The Drosophila ovary: an active stem cell community. Cell Res. 2007;17:15–25. - PubMed
-
- Xie T, Spradling A. C. decapentaplegic is essential for the maintenance and division of germline stem cells in the Drosophila ovary. Cell. 1998;94:251–260. - PubMed
-
- Xie T, Spradling A. C. A niche maintaining germ line stem cells in the Drosophila ovary. Science. 2000;290:328–330. - PubMed
-
- Chen D, McKearin D. Dpp signaling silences bam transcription directly to establish asymmetric divisions of germline stem cells. Curr Biol. 2003;13:1786–1791. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

