Function and pharmacology of spinally-projecting sympathetic pre-autonomic neurones in the paraventricular nucleus of the hypothalamus
- PMID: 22131936
- PMCID: PMC3131718
- DOI: 10.2174/157015911795596531
Function and pharmacology of spinally-projecting sympathetic pre-autonomic neurones in the paraventricular nucleus of the hypothalamus
Abstract
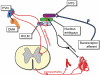
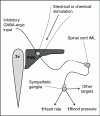
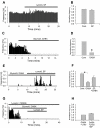
The paraventricular nucleus (PVN) of the hypothalamus has been described as the "autonomic master controller". It co-ordinates critical physiological responses through control of the hypothalamic-pituitary-adrenal (HPA)-axis, and by modulation of the sympathetic and parasympathetic branches of the central nervous system. The PVN comprises several anatomical subdivisions, including the parvocellular/ mediocellular subdivision, which contains neurones projecting to the medulla and spinal cord. Consensus indicates that output from spinally-projecting sympathetic pre-autonomic neurones (SPANs) increases blood pressure and heart rate, and dysfunction of these neurones has been directly linked to elevated sympathetic activity during heart failure. The influence of spinally-projecting SPANs on cardiovascular function high-lights their potential as targets for future therapeutic drug development. Recent studies have demonstrated pharmacological control of these spinally-projecting SPANs with glutamate, GABA, nitric oxide, neuroactive steroids and a number of neuropeptides (including angiotensin, substance P, and corticotrophin-releasing factor). The underlying mechanism of control appears to be a state of tonic inhibition by GABA, which is then strengthened or relieved by the action of other modulators. The physiological function of spinally-projecting SPANs has been subject to some debate, and they may be involved in physiological stress responses, blood volume regulation, glucose regulation, thermoregulation and/or circadian rhythms. This review describes the pharmacology of PVN spinally-projecting SPANs and discusses their likely roles in cardiovascular control.
Keywords: Blood pressure; GABA; PVN; angiotensin; cardiovascular; hypothalamus; neuropeptides; oxytocin; paraventricular nucleus; parvocellular mediocellular; penile erection; pharmacology.; substance P; sympathetic; tachykinin; vasopressin.
Figures



Similar articles
-
A role for the paraventricular nucleus of the hypothalamus in the autonomic control of heart and kidney.Exp Physiol. 2005 Mar;90(2):169-73. doi: 10.1113/expphysiol.2004.029041. Epub 2004 Dec 16. Exp Physiol. 2005. PMID: 15604110 Review.
-
Neurochemistry of the paraventricular nucleus of the hypothalamus: implications for cardiovascular regulation.J Chem Neuroanat. 2009 Nov;38(3):197-208. doi: 10.1016/j.jchemneu.2009.03.005. Epub 2009 Mar 28. J Chem Neuroanat. 2009. PMID: 19778682 Review.
-
Inhibition by alpha-tetrahydrodeoxycorticosterone (THDOC) of pre-sympathetic parvocellular neurones in the paraventricular nucleus of rat hypothalamus.Br J Pharmacol. 2006 Nov;149(5):600-7. doi: 10.1038/sj.bjp.0706911. Epub 2006 Sep 25. Br J Pharmacol. 2006. PMID: 17001301 Free PMC article.
-
Cardiac sympatho-excitatory action of PVN-spinal oxytocin neurones.Auton Neurosci. 2009 May 11;147(1-2):80-5. doi: 10.1016/j.autneu.2009.01.013. Epub 2009 Mar 9. Auton Neurosci. 2009. PMID: 19269259
-
NK1-receptor-expressing paraventricular nucleus neurones modulate daily variation in heart rate and stress-induced changes in heart rate variability.Physiol Rep. 2014 Dec 3;2(12):e12207. doi: 10.14814/phy2.12207. Print 2014 Dec 1. Physiol Rep. 2014. PMID: 25472606 Free PMC article.
Cited by
-
HSP-70-Mediated Hyperbaric Oxygen Reduces Brain and Pulmonary Edema and Cognitive Deficits in Rats in a Simulated High-Altitude Exposure.Biomed Res Int. 2018 Nov 1;2018:4608150. doi: 10.1155/2018/4608150. eCollection 2018. Biomed Res Int. 2018. PMID: 30515398 Free PMC article.
-
Functional Organization of the Sympathetic Pathways Controlling the Pupil: Light-Inhibited and Light-Stimulated Pathways.Front Neurol. 2018 Dec 18;9:1069. doi: 10.3389/fneur.2018.01069. eCollection 2018. Front Neurol. 2018. PMID: 30619035 Free PMC article. Review.
-
Oxytocin participates on the effects of vasoactive intestinal peptide on food intake and plasma parameters.Mol Cell Biochem. 2018 Jan;437(1-2):177-183. doi: 10.1007/s11010-017-3106-x. Epub 2017 Jul 27. Mol Cell Biochem. 2018. PMID: 28752412
-
Preoptic BRS3 neurons increase body temperature and heart rate via multiple pathways.Cell Metab. 2021 Jul 6;33(7):1389-1403.e6. doi: 10.1016/j.cmet.2021.05.001. Epub 2021 May 25. Cell Metab. 2021. PMID: 34038711 Free PMC article.
-
Activation of the hypothalamic paraventricular nucleus by acute intermittent hypoxia: Implications for sympathetic long-term facilitation neuroplasticity.Exp Neurol. 2019 Apr;314:1-8. doi: 10.1016/j.expneurol.2018.12.011. Epub 2018 Dec 31. Exp Neurol. 2019. PMID: 30605624 Free PMC article.
References
-
- Swanson LW, Sawchenko PE, Wiegand SJ, Price JL. Separate neurons in the paraventricular nucleus project to the median-eminence and to the medulla or spinal-cord. Brain Res. 1980;198:190–195. - PubMed
-
- Palkovits M. Interconnections between the neuroendocrine hypothalamus and the central autonomic system. Front. Neuroendocrinol. 1999;20:270–295. - PubMed
-
- Swanson L, Kuypers H. The paraventricular nucleus of the hypothalamus: Cytoarchitectonic subdivisions and organization of projections to the pituitary, dorsal vagal complex, and spinal cord as demonstrated by retrograde fluorescence double-labeling methods. J. Comp. Neurol. 1980;194:555–570. - PubMed
-
- Swanson LW, Sawchenko PE. Hypothalamic integration - organization of the paraventricular and supraoptic nuclei. Ann. Rev. Neurosci. 1983;6:269–324. - PubMed
-
- Herman JP, Cullinan WE. Neurocircuitry of stress: Central control of the hypothalamo-pituitary-adrenocortical axis. Trends Neurosci. 1997;20:78–84. - PubMed
LinkOut - more resources
Full Text Sources
