Simultaneous parasympathetic and sympathetic activation reveals altered autonomic control of heart rate, vascular tension, and epinephrine release in anesthetized hypertensive rats
- PMID: 22131984
- PMCID: PMC3222849
- DOI: 10.3389/fneur.2011.00071
Simultaneous parasympathetic and sympathetic activation reveals altered autonomic control of heart rate, vascular tension, and epinephrine release in anesthetized hypertensive rats
Abstract
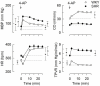
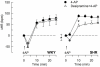
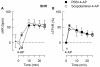
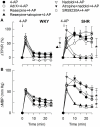
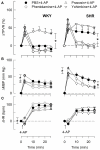
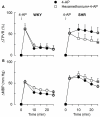
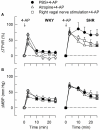
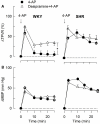
Sympathetic hyperactivity and parasympathetic insufficiency characterize blood pressure (BP) control in genetic hypertension. This shift is difficult to investigate in anesthetized rats. Here we present a pharmacological approach to simultaneously provoke sympathetic and parasympathetic transmitter release, and identify their respective roles in the concomitant cardiovascular response. To stimulate transmitter release in anesthetized normotensive (WKY) and spontaneously hypertensive rats (SHR), we injected intravenously 4-aminopyridine (4-AP), a voltage-sensitive K(+) channel (K(V)) inhibitor. A femoral artery catheter monitored BP, an ascending aorta flow-probe recorded cardiac output and heart rate (HR). Total peripheral vascular resistance (TPVR) was calculated. 4-AP-induced an immediate, atropine (muscarinic antagonist)- and hexamethonium (ganglion blocker)-sensitive bradycardia in WKY, and in both strains, a subsequent, sustained tachycardia, and norepinephrine but not epinephrine release. Reserpine (sympatholytic), nadolol (β-adrenoceptor antagonist) or right vagal nerve stimulation eliminated the late tachycardia, adrenalectomy, scopolamine (central muscarinic antagonist) or hexamethonium did not. 4-AP increased TPVR, transiently in WKY but sustained in SHR. Yohimbine (α(2)-adrenoceptor antagonist) prevented the TPVR down-regulation in WKY. Reserpine and prazosin (α(1)-adrenoceptor antagonist) eliminated the late vasoconstriction in SHR. Plasma epinephrine overflow increased in nadolol-treated SHR. Through inhibition of K(V), 4-AP activated parasympathetic ganglion transmission and peripheral, neuronal norepinephrine release. The sympathetic component dominated the 4-AP-HR-response in SHR. α(2)-adrenoceptor-dependent vasodilatation opposed norepinephrine-induced α(1)-adrenergic vasoconstriction in WKY, but not SHR. A βAR-activated, probably vagal afferent mechanism, hampered epinephrine secretion in SHR. Thus, 4-AP activated the autonomic system and exposed mechanisms relevant to hypertensive disease.
Keywords: 4-aminopyridine; adrenal; epinephrine; heart rate; norepinephrine; parasympathetic nerves; sympathetic nerves; total peripheral vascular resistance.
Figures


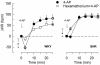

References
LinkOut - more resources
Full Text Sources

