Improving Clinical Trial Participant Tracking Tools Using Knowledge-anchored Design Methodologies
- PMID: 22132037
- PMCID: PMC3225206
- DOI: 10.4338/ACI-2010-02-RA-0012
Improving Clinical Trial Participant Tracking Tools Using Knowledge-anchored Design Methodologies
Abstract
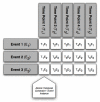
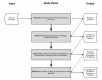
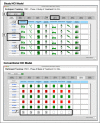
OBJECTIVE: Rigorous human-computer interaction (HCI) design methodologies have not traditionally been applied to the development of clinical trial participant tracking (CTPT) tools. Given the frequent us of iconic HCI models in CTPTs, and prior evidence of usability problems associated with the use of ambiguous icons in complex interfaces, such approaches may be problematic. Presentation Discovery (PD), a knowledge-anchored HCI design method, has been previously demonstrated to improve the design of iconic HCI models. In this study, we compare the usability of a CTPT HCI model designed using PD and an intuitively designed CTPT HCI model. METHODS: An iconic CPTP HCI model was created using PD. The PD-generated and an existing iconic CTPT HCI model were subjected to usability testing, with an emphasis on task accuracy and completion times. Study participants also completed a qualitative survey instrument to evaluate subjective satisfaction with the two models. RESULTS: CTPT end-users reliably and reproducibly agreed on the visual manifestation and semantics of prototype graphics generated using PD. The performance of the PD-generated iconic HCI model was equivalent to an existing HCI model for tasks at multiple levels of complexity, and in some cases superior. This difference was particularly notable when tasks required an understanding of the semantic meanings of multiple icons. CONCLUSION: The use of PD to design an iconic CTPT HCI model generated beneficial results and improved end-user subjective satisfaction, while reducing task completion time. Such results are desirable in information and time intensive domains, such as clinical trials management.
Figures

Similar articles
-
Optimizing Clinical Decision Support System Functionality by Leveraging Specific Human-Computer Interaction Elements: Insights From a Systematic Review.JMIR Hum Factors. 2025 May 6;12:e69333. doi: 10.2196/69333. JMIR Hum Factors. 2025. PMID: 40327851 Free PMC article. Review.
-
HCI-modelling for improving the clinical usability of digital health technologies.Methods. 2024 Jul;227:60-77. doi: 10.1016/j.ymeth.2024.04.019. Epub 2024 May 9. Methods. 2024. PMID: 38729456
-
Iconic hyperlinks on e-commerce websites.Appl Ergon. 2007 Jan;38(1):65-9. doi: 10.1016/j.apergo.2006.01.007. Epub 2006 Mar 14. Appl Ergon. 2007. PMID: 16537075 Clinical Trial.
-
Assessing the Usability of a Clinical Decision Support System: Heuristic Evaluation.JMIR Hum Factors. 2022 May 10;9(2):e31758. doi: 10.2196/31758. JMIR Hum Factors. 2022. PMID: 35536613 Free PMC article.
-
USER FRUSTRATION IN HIT INTERFACES: EXPLORING PAST HCI RESEARCH FOR A BETTER UNDERSTANDING OF CLINICIANS' EXPERIENCES.AMIA Annu Symp Proc. 2015 Nov 5;2015:1008-17. eCollection 2015. AMIA Annu Symp Proc. 2015. PMID: 26958238 Free PMC article. Review.
Cited by
-
Validating the information technology (IT) implementation framework to Implement mHealth technology for consumers: A case study of the Sense2Quit app for smoking cessation.Int J Med Inform. 2025 Oct;202:105977. doi: 10.1016/j.ijmedinf.2025.105977. Epub 2025 May 20. Int J Med Inform. 2025. PMID: 40413979
-
The TOKEn project: knowledge synthesis for in silico science.J Am Med Inform Assoc. 2011 Dec;18 Suppl 1(Suppl 1):i125-31. doi: 10.1136/amiajnl-2011-000434. Epub 2011 Oct 7. J Am Med Inform Assoc. 2011. PMID: 21984589 Free PMC article.
-
Usage Metrics of Web-Based Interventions Evaluated in Randomized Controlled Trials: Systematic Review.J Med Internet Res. 2020 Apr 16;22(4):e15474. doi: 10.2196/15474. J Med Internet Res. 2020. PMID: 32297870 Free PMC article.
-
Advancing user experience research to facilitate and enable patient-centered research: current state and future directions.EGEMS (Wash DC). 2013 Jun 20;1(1):1026. doi: 10.13063/2327-9214.1026. eCollection 2013. EGEMS (Wash DC). 2013. PMID: 25848566 Free PMC article.
-
Perspectives on the design and methodology of periconceptional nutrient supplementation trials.Trials. 2016 Jan 30;17:58. doi: 10.1186/s13063-015-1124-0. Trials. 2016. PMID: 26833080 Free PMC article.
References
-
- Chung TK, Kukafka R, Johnson SB. Reengineering clinical research with informatics. J Investig Med 2006Sep 1; 54(6): 327-333 - PubMed
-
- Payne PR, Johnson SB, Starren JB, Tilson HH, Dowdy D. Breaking the translational barriers: the value of integrating biomedical informatics and translational research. J Investig Med 2005May; 53(4): 192-200 - PubMed
-
- Sung NS, Crowley WF, Jr., Genel M, Salber P, Sandy L, Sherwood LM, et al. Central challenges facing the national clinical research enterprise. JAMA 2003Mar 12; 289(10): 1278-1287 - PubMed
-
- Khan SA, Kukafka R, Payne PR, Bigger JT, Johnson SB. A day in the life of a clinical research coordinator: observations from community practice settings. Medinfo 2007; 12(Pt 1): 247-251 - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

