Structure prediction and validation of an affibody engineered for cell-specific nucleic acid targeting
- PMID: 22132056
- PMCID: PMC3065588
- DOI: 10.1007/s11693-011-9074-7
Structure prediction and validation of an affibody engineered for cell-specific nucleic acid targeting
Abstract
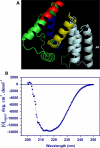
Cell-penetrating peptides comprising cloned epitopes that contribute to membrane transduction, DNA-binding and cell targeting functions are known to facilitate nucleic acid delivery. Using the ITASSER software, we predicted the 3-D structure of a well characterized and efficient transfecting cell-penetrating peptide, namely TAT-Mu and its derivative TAT-Mu-AF protein that harbors a targeting ligand, the HER2-binding affibody. Our model predicts TAT-Mu-AF fusion protein as primarily comprising α-helices. The affibody in TAT-Mu-AF is predicted as a 3-helical domain that is distinct from the TAT-Mu domain. Its positioning in three-dimensional structure is oriented in a manner that possibly favors interactions with receptor and facilitates transport to the target site. The linker region between TAT-Mu and the affibody is also predicted as a helix that is likely to stabilize the overall fold of the TAT-Mu-AF complex. Further, the evaluation of secondary structure of the designed TAT-Mu-AF fusion protein by circular dichroism is in support of our predictions.
Keywords: 3-D modelling; Affibody; CPPs; Cell-targeting; Nucleic acid delivery; TAT fusions; iTASSER.
Figures
Similar articles
-
Targeting human epidermal growth factor receptor 2 by a cell-penetrating peptide-affibody bioconjugate.Biomaterials. 2012 Mar;33(8):2570-82. doi: 10.1016/j.biomaterials.2011.12.003. Epub 2011 Dec 20. Biomaterials. 2012. PMID: 22192536
-
Recombinant fusion proteins TAT-Mu, Mu and Mu-Mu mediate efficient non-viral gene delivery.J Gene Med. 2007 Apr;9(4):275-86. doi: 10.1002/jgm.1014. J Gene Med. 2007. PMID: 17397090
-
Site-specific radiometal labeling and improved biodistribution using ABY-027, a novel HER2-targeting affibody molecule-albumin-binding domain fusion protein.J Nucl Med. 2013 Jun;54(6):961-8. doi: 10.2967/jnumed.112.110700. Epub 2013 Mar 25. J Nucl Med. 2013. PMID: 23528382
-
Transport molecules using reverse sequence HIV-Tat polypeptides: not just any old Tat? (WO200808225).Expert Opin Ther Pat. 2009 Sep;19(9):1329-33. doi: 10.1517/17530050902824829. Expert Opin Ther Pat. 2009. PMID: 19555160 Review.
-
In vitro and in vivo delivery of therapeutic proteins using cell penetrating peptides.Peptides. 2017 Jan;87:50-63. doi: 10.1016/j.peptides.2016.11.011. Epub 2016 Nov 22. Peptides. 2017. PMID: 27887988 Review.
Cited by
-
Intratumoral chemotherapy for lung cancer: re-challenge current targeted therapies.Drug Des Devel Ther. 2013 Jul 18;7:571-83. doi: 10.2147/DDDT.S46393. Print 2013. Drug Des Devel Ther. 2013. PMID: 23898222 Free PMC article.
-
Differential Evolutionary Selection and Natural Evolvability Observed in ALT Proteins of Human Filarial Parasites.PLoS One. 2016 Feb 18;11(2):e0148611. doi: 10.1371/journal.pone.0148611. eCollection 2016. PLoS One. 2016. PMID: 26890364 Free PMC article.
-
A novel B- and helper T-cell epitopes-based prophylactic vaccine against Echinococcus granulosus.Bioimpacts. 2018;8(1):39-52. doi: 10.15171/bi.2018.06. Epub 2017 Dec 20. Bioimpacts. 2018. PMID: 29713601 Free PMC article.
References
-
- DeLano WL (2002) The PyMOL molecular graphics system. DeLano Scientific, San Carlos, CA, USA. http://www.pymol.org
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

