Evaluation of high-throughput PCR and microarray-based assay in conjunction with automated DNA extraction instruments for diagnosis of sepsis
- PMID: 22132076
- PMCID: PMC3222647
- DOI: 10.1371/journal.pone.0026655
Evaluation of high-throughput PCR and microarray-based assay in conjunction with automated DNA extraction instruments for diagnosis of sepsis
Abstract
Background: High incidence of septic patients increases the pressure of faster and more reliable bacterial identification methods to adapt patient management towards focused and effective treatment options. The aim of this study was to assess two automated DNA extraction solutions with the PCR and microarray-based assay to enable rapid and reliable detection and speciation of causative agents in the diagnosis of sepsis.
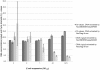
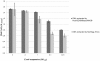
Methodology/principal findings: We evaluated two automated DNA instruments NucliSENS® easyMAG® and NorDiag Arrow for the preparation of blood culture samples. A set of 91 samples flagged as positive during incubation was analyzed prospectively with the high-throughput generation of Prove-it™ Sepsis assay designed to identify over 60 gram-negative and gram-positive bacterial species as well as methicillin resistance marker from a blood culture. Bacterial findings were accurately reported from 77 blood culture samples, whereas 14 samples were reported as negative, containing bacteria not belonging to the pathogen panel of the assay. No difference was observed between the performance of NorDiag Arrow or NucliSENS® easyMAG® with regard to the result reporting of Prove-it™ Sepsis. In addition, we also assessed the quality and quantity of DNA extracted from the clinical Escherichia coli isolate with DNA extraction instruments. We observed only minor differences between the two instruments.
Conclusions: Use of automated and standardized sample preparation methods together with rapid, multiplex pathogen detection offers a strategy to speed up reliably the diagnostics of septic patients. Both tested DNA extraction devices were shown to be feasible for blood culture samples and the Prove-it™ Sepsis assay, providing an accurate identification of pathogen within 4.5 hours when the detected pathogen was in the repertoire of the test.
Conflict of interest statement
Figures
Similar articles
-
Commercial multiplex technologies for the microbiological diagnosis of sepsis.Mol Diagn Ther. 2013 Aug;17(4):221-31. doi: 10.1007/s40291-013-0037-4. Mol Diagn Ther. 2013. PMID: 23636778 Free PMC article. Review.
-
Assessment of a semi-automated protocol for multiplex analysis of sepsis-causing bacteria with spiked whole blood samples.Microbiologyopen. 2013 Apr;2(2):284-92. doi: 10.1002/mbo3.69. Epub 2013 Feb 18. Microbiologyopen. 2013. PMID: 23417871 Free PMC article.
-
Evaluation of the Punch-it™ NA-Sample kit for detecting microbial DNA in blood culture bottles using PCR-reverse blot hybridization assay.J Microbiol Methods. 2016 Sep;128:24-30. doi: 10.1016/j.mimet.2016.06.001. Epub 2016 Jun 2. J Microbiol Methods. 2016. PMID: 27263831
-
Clinical evaluation of commercial nucleic acid amplification tests in patients with suspected sepsis.BMC Infect Dis. 2015 Apr 28;15:199. doi: 10.1186/s12879-015-0938-4. BMC Infect Dis. 2015. PMID: 25928122 Free PMC article.
-
Molecular microbiological methods in the diagnosis of neonatal sepsis.Expert Rev Anti Infect Ther. 2010 Sep;8(9):1037-48. doi: 10.1586/eri.10.89. Expert Rev Anti Infect Ther. 2010. PMID: 20818947 Free PMC article. Review.
Cited by
-
Efficacy of a novel PCR- and microarray-based method in diagnosis of a prosthetic joint infection.Acta Orthop. 2014 Apr;85(2):165-70. doi: 10.3109/17453674.2014.889978. Epub 2014 Feb 25. Acta Orthop. 2014. PMID: 24564748 Free PMC article.
-
Early diagnosis of resistant pathogens: how can it improve antimicrobial treatment?Virulence. 2013 Feb 15;4(2):172-84. doi: 10.4161/viru.23326. Epub 2013 Jan 9. Virulence. 2013. PMID: 23302786 Free PMC article. Review.
-
A novel flow cytometry assay based on bacteriophage-derived proteins for Staphylococcus detection in blood.Sci Rep. 2020 Apr 10;10(1):6260. doi: 10.1038/s41598-020-62533-7. Sci Rep. 2020. PMID: 32277078 Free PMC article.
-
Commercial multiplex technologies for the microbiological diagnosis of sepsis.Mol Diagn Ther. 2013 Aug;17(4):221-31. doi: 10.1007/s40291-013-0037-4. Mol Diagn Ther. 2013. PMID: 23636778 Free PMC article. Review.
-
Diagnostic Accuracy of Procalcitonin Compared to C-Reactive Protein and Interleukin 6 in Recognizing Gram-Negative Bloodstream Infection: A Meta-Analytic Study.Dis Markers. 2020 Jan 23;2020:4873074. doi: 10.1155/2020/4873074. eCollection 2020. Dis Markers. 2020. PMID: 32076461 Free PMC article.
References
-
- Ebrahim GJ. Sepsis, septic shock and the systemic inflammatory response syndrome. J Trop Pediatr. 2011;57:77–79. - PubMed
-
- Garey KW, Rege M, Pai MP, Mingo DE, Suda KJ, et al. Time to initiation of fluconazole therapy impacts mortality in patients with candidemia: a multi-institutional study. Clin Infect Dis. 2006;43:25–31. - PubMed
-
- Kumar A, Roberts D, Wood KE, Light B, Parrillo JE, et al. Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock. Crit Care Med. 2006;34:1589–96. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

