Enhanced Hsp70 expression protects against acute lung injury by modulating apoptotic pathways
- PMID: 22132083
- PMCID: PMC3223157
- DOI: 10.1371/journal.pone.0026956
Enhanced Hsp70 expression protects against acute lung injury by modulating apoptotic pathways
Abstract
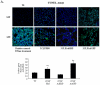
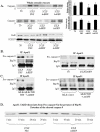
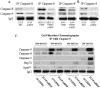
The Acute respiratory distress syndrome (ARDS) is a highly lethal inflammatory lung disorder. Apoptosis plays a key role in its pathogenesis. We showed that an adenovirus expressing the 70 kDa heat shock protein Hsp70 (AdHSP) protected against sepsis-induced lung injury. In this study we tested the hypothesis that AdHSP attenuates apoptosis in sepsis-induced lung injury. Sepsis was induced in rats via cecal ligation and double puncture (2CLP). At the time of 2CLP PBS, AdHSP or AdGFP (an adenoviral vector expressing green fluorescent protein) were injected into the tracheas of septic rats. 48 hours later, lungs were isolated. One lung was fixed for TUNEL staining and immunohistochemistry. The other was homogenized to isolate cytosolic and nuclear protein. Immunoblotting, gel filtration and co-immunoprecipitation were performed in these extracts. In separate experiments MLE-12 cells were incubated with medium, AdHSP or AdGFP. Cells were stimulated with TNFα. Cytosolic and nuclear proteins were isolated. These were subjected to immunoblotting, co-immunoprecipitation and a caspase-3 activity assay. TUNEL assay demonstrated that AdHSP reduced alveolar cell apoptosis. This was confirmed by immunohistochemical detection of caspase 3 abundance. In lung isolated from septic animals, immunoblotting, co-immunoprecipitation and gel filtration studies revealed an increase in cytoplasmic complexes containing caspases 3, 8 and 9. AdHSP disrupted these complexes. We propose that Hsp70 impairs apoptotic cellular pathways via interactions with caspases. Disruption of large complexes resulted in stabilization of lower molecular weight complexes, thereby, reducing nuclear caspase-3. Prevention of apoptosis in lung injury may preserve alveolar cells and aid in recovery.
Conflict of interest statement
Figures


Similar articles
-
Moderate Fever Cycles as a Potential Mechanism to Protect the Respiratory System in COVID-19 Patients.Front Med (Lausanne). 2020 Sep 11;7:564170. doi: 10.3389/fmed.2020.564170. eCollection 2020. Front Med (Lausanne). 2020. PMID: 33043037 Free PMC article. Review.
-
Enhanced expression of 70-kilodalton heat shock protein limits cell division in a sepsis-induced model of acute respiratory distress syndrome.Crit Care Med. 2008 Jan;36(1):246-55. doi: 10.1097/01.CCM.0000295473.56522.EF. Crit Care Med. 2008. PMID: 17989570 Free PMC article.
-
Enhanced heat shock protein 70 expression alters proteasomal degradation of IkappaB kinase in experimental acute respiratory distress syndrome.Crit Care Med. 2007 Sep;35(9):2128-38. doi: 10.1097/01.ccm.0000278915.78030.74. Crit Care Med. 2007. PMID: 17855826
-
Adenoviral transfer of HSP-70 into pulmonary epithelium ameliorates experimental acute respiratory distress syndrome.J Clin Invest. 2002 Sep;110(6):801-6. doi: 10.1172/JCI15888. J Clin Invest. 2002. PMID: 12235111 Free PMC article.
-
Reduction of caspase-8 and -9 cleavage is associated with increased c-FLIP and increased binding of Apaf-1 and Hsp70 after neonatal hypoxic/ischemic injury in mice overexpressing Hsp70.Stroke. 2006 Feb;37(2):507-12. doi: 10.1161/01.STR.0000199057.00365.20. Epub 2006 Jan 5. Stroke. 2006. PMID: 16397188
Cited by
-
Quantitative proteomics of heat-treated human cells show an across-the-board mild depletion of housekeeping proteins to massively accumulate few HSPs.Cell Stress Chaperones. 2015 Jul;20(4):605-20. doi: 10.1007/s12192-015-0583-2. Epub 2015 Apr 8. Cell Stress Chaperones. 2015. PMID: 25847399 Free PMC article.
-
HSP70 Is a Critical Regulator of HSP90 Inhibitor's Effectiveness in Preventing HCl-Induced Chronic Lung Injury and Pulmonary Fibrosis.Int J Mol Sci. 2024 Feb 5;25(3):1920. doi: 10.3390/ijms25031920. Int J Mol Sci. 2024. PMID: 38339194 Free PMC article.
-
Multi-layered molecular mechanisms of polypeptide holding, unfolding and disaggregation by HSP70/HSP110 chaperones.Front Mol Biosci. 2015 Jun 5;2:29. doi: 10.3389/fmolb.2015.00029. eCollection 2015. Front Mol Biosci. 2015. PMID: 26097841 Free PMC article. Review.
-
Unraveling the Impact of Dab1 Gene Silencing on the Expression of Autophagy Markers in Lung Development.Life (Basel). 2024 Feb 28;14(3):316. doi: 10.3390/life14030316. Life (Basel). 2024. PMID: 38541642 Free PMC article.
-
Moderate Fever Cycles as a Potential Mechanism to Protect the Respiratory System in COVID-19 Patients.Front Med (Lausanne). 2020 Sep 11;7:564170. doi: 10.3389/fmed.2020.564170. eCollection 2020. Front Med (Lausanne). 2020. PMID: 33043037 Free PMC article. Review.
References
-
- Rubenfeld GD, Caldwell E, Peabody E, Weaver J, Martin DP, et al. Incidence and outcomes of acute lung injury. N Engl J Med. 2005;353:1685–1693. - PubMed
-
- Ware LB, Matthay MA. The acute respiratory distress syndrome. N Engl J Med. 2000;342:1334–1349. - PubMed
-
- Brower RG, Lanken PN, MacIntyre N, Matthay MA, Morris A, et al. Higher versus lower positive end-expiratory pressures in patients with the acute respiratory distress syndrome. N Engl J Med. 2004;351:327–336. - PubMed
-
- Ware LB, Matthay MA. Alveolar fluid clearance is impaired in the majority of patients with acute lung injury and the acute respiratory distress syndrome. Am J Respir Crit Care Med. 2001;163:1376–1383. - PubMed
-
- Steinberg H, Greenwald RA, Sciubba J, Das DK. The effect of oxygen-derived free radicals on pulmonary endothelial cell function in the isolated perfused rat lung. Exp Lung Res. 1982;3(2):163–173. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

