Generation of immortal cell lines from the adult pituitary: role of cAMP on differentiation of SOX2-expressing progenitor cells to mature gonadotropes
- PMID: 22132145
- PMCID: PMC3221660
- DOI: 10.1371/journal.pone.0027799
Generation of immortal cell lines from the adult pituitary: role of cAMP on differentiation of SOX2-expressing progenitor cells to mature gonadotropes
Abstract
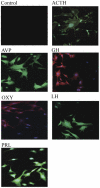
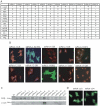
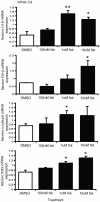
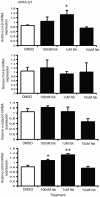
The pituitary is a complex endocrine tissue composed of a number of unique cell types distinguished by the expression and secretion of specific hormones, which in turn control critical components of overall physiology. The basic function of these cells is understood; however, the molecular events involved in their hormonal regulation are not yet fully defined. While previously established cell lines have provided much insight into these regulatory mechanisms, the availability of representative cell lines from each cell lineage is limited, and currently none are derived from adult pituitary. We have therefore used retroviral transfer of SV40 T-antigen to mass immortalize primary pituitary cell culture from an adult mouse. We have generated 19 mixed cell cultures that contain cells from pituitary cell lineages, as determined by RT-PCR analysis and immunocytochemistry for specific hormones. Some lines expressed markers associated with multipotent adult progenitor cells or transit-amplifying cells, including SOX2, nestin, S100, and SOX9. The progenitor lines were exposed to an adenylate cyclase activator, forskolin, over 7 days and were induced to differentiate to a more mature gonadotrope cell, expressing significant levels of α-subunit, LHβ, and FSHβ mRNAs. Additionally, clonal populations of differentiated gonadotropes were exposed to 30 nM gonadotropin-releasing hormone and responded appropriately with a significant increase in α-subunit and LHβ transcription. Further, exposure of the lines to a pulse paradigm of GnRH, in combination with 17β-estradiol and dexamethasone, significantly increased GnRH receptor mRNA levels. This array of adult-derived pituitary cell models will be valuable for both studies of progenitor cell characteristics and modulation, and the molecular analysis of individual pituitary cell lineages.
Conflict of interest statement
Figures


Similar articles
-
Possible role of PACAP and its PAC1 receptor in the differential regulation of pituitary LHbeta- and FSHbeta-subunit gene expression by pulsatile GnRH stimulation.Biol Reprod. 2013 Feb 14;88(2):35. doi: 10.1095/biolreprod.112.105601. Print 2013 Feb. Biol Reprod. 2013. PMID: 23197164 Review.
-
Regulation of gonadotropin subunit genes in tilapia.Comp Biochem Physiol B Biochem Mol Biol. 2001 Jun;129(2-3):489-502. doi: 10.1016/s1096-4959(01)00345-1. Comp Biochem Physiol B Biochem Mol Biol. 2001. PMID: 11399484 Review.
-
Cell-specific transcriptional regulation of follicle-stimulating hormone-beta by activin and gonadotropin-releasing hormone in the LbetaT2 pituitary gonadotrope cell model.Endocrinology. 2001 Jun;142(6):2284-95. doi: 10.1210/endo.142.6.8185. Endocrinology. 2001. PMID: 11356674
-
PACAP induces FSHβ gene expression via EPAC.Mol Cell Endocrinol. 2019 Jul 15;492:110438. doi: 10.1016/j.mce.2019.04.018. Epub 2019 Apr 26. Mol Cell Endocrinol. 2019. PMID: 31034837 Free PMC article.
-
Growth hormone-releasing hormone and glucocorticoids determine the balance between luteinising hormone (LH) beta- and LH beta/follicle-stimulating hormone beta-positive gonadotrophs and somatotrophs in the 14-day-old rat pituitary tissue in aggregate cell culture.J Neuroendocrinol. 2008 May;20(5):535-48. doi: 10.1111/j.1365-2826.2008.01698.x. Epub 2008 Mar 15. J Neuroendocrinol. 2008. PMID: 18363807
Cited by
-
The regulation of reproductive neuroendocrine function by insulin and insulin-like growth factor-1 (IGF-1).Front Neuroendocrinol. 2014 Oct;35(4):558-72. doi: 10.1016/j.yfrne.2014.05.007. Epub 2014 Jun 12. Front Neuroendocrinol. 2014. PMID: 24929098 Free PMC article. Review.
-
Establishment of a protocol to extend the lifespan of human hormone-secreting pituitary adenoma cells.Endocrine. 2018 Jan;59(1):102-108. doi: 10.1007/s12020-017-1305-6. Epub 2017 Apr 26. Endocrine. 2018. PMID: 28447256
-
The relationship between basal and regulated Gnrhr expression in rodent pituitary gonadotrophs.Mol Cell Endocrinol. 2016 Dec 5;437:302-311. doi: 10.1016/j.mce.2016.08.040. Epub 2016 Aug 26. Mol Cell Endocrinol. 2016. PMID: 27569529 Free PMC article.
-
Autoantibodies involved in primary and secondary adrenal insufficiency following treatment with immune checkpoint inhibitors.Immunooncol Technol. 2023 Feb 11;17:100374. doi: 10.1016/j.iotech.2023.100374. eCollection 2023 Mar. Immunooncol Technol. 2023. PMID: 36937704 Free PMC article. Review.
-
Direct and Indirect Effects of Sex Steroids on Gonadotrope Cell Plasticity in the Teleost Fish Pituitary.Front Endocrinol (Lausanne). 2020 Dec 7;11:605068. doi: 10.3389/fendo.2020.605068. eCollection 2020. Front Endocrinol (Lausanne). 2020. PMID: 33365013 Free PMC article. Review.
References
-
- Ooi GT, Tawadros N, Escalona RM. Pituitary cell lines and their endocrine applications. Mol Cell Endocrinol. 2004;228:1–21. - PubMed
-
- Belsham DD, Cai F, Cui H, Smukler SR, Salapatek AM, et al. Generation of a phenotypic array of hypothalamic neuronal cell models to study complex neuroendocrine disorders. Endocrinology. 2004;145:393–400. - PubMed
-
- Belsham DD, Fick LJ, Dalvi PS, Centeno ML, Chalmers JA, et al. Faseb J; 2009. Ciliary neurotrophic factor recruitment of glucagon-like peptide-1 mediates neurogenesis, allowing immortalization of adult murine hypothalamic neurons. - PubMed
-
- Dhillon SS, Kim GL, Belsham DD. Neuroendocrine gene regulation in hypothalamic cell lines. Open Neuroendocrin J. 2010;3:6–15.
-
- Mayer CM, Fick LJ, Gingerich S, Belsham DD. Hypothalamic cell lines to investigate neuroendocrine control mechanisms. Front Neuroendocrinol. 2009;30:405–423. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

