Phenotypes of non-attached Pseudomonas aeruginosa aggregates resemble surface attached biofilm
- PMID: 22132176
- PMCID: PMC3221681
- DOI: 10.1371/journal.pone.0027943
Phenotypes of non-attached Pseudomonas aeruginosa aggregates resemble surface attached biofilm
Abstract
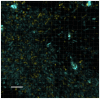
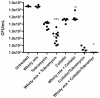
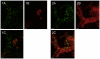
For a chronic infection to be established, bacteria must be able to cope with hostile conditions such as low iron levels, oxidative stress, and clearance by the host defense, as well as antibiotic treatment. It is generally accepted that biofilm formation facilitates tolerance to these adverse conditions. However, microscopic investigations of samples isolated from sites of chronic infections seem to suggest that some bacteria do not need to be attached to surfaces in order to establish chronic infections. In this study we employed scanning electron microscopy, confocal laser scanning microscopy, RT-PCR as well as traditional culturing techniques to study the properties of Pseudomonas aeruginosa aggregates. We found that non-attached aggregates from stationary-phase cultures have comparable growth rates to surface attached biofilms. The growth rate estimations indicated that, independently of age, both aggregates and flow-cell biofilm had the same slow growth rate as a stationary phase shaking cultures. Internal structures of the aggregates matrix components and their capacity to survive otherwise lethal treatments with antibiotics (referred to as tolerance) and resistance to phagocytes were also found to be strikingly similar to flow-cell biofilms. Our data indicate that the tolerance of both biofilms and non-attached aggregates towards antibiotics is reversible by physical disruption. We provide evidence that the antibiotic tolerance is likely to be dependent on both the physiological states of the aggregates and particular matrix components. Bacterial surface-attachment and subsequent biofilm formation are considered hallmarks of the capacity of microbes to cause persistent infections. We have observed non-attached aggregates in the lungs of cystic fibrosis patients; otitis media; soft tissue fillers and non-healing wounds, and we propose that aggregated cells exhibit enhanced survival in the hostile host environment, compared with non-aggregated bacterial populations.
Conflict of interest statement
Figures


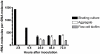





References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

