Switch of voltage-gated K+ channel expression in the plasma membrane of chondrogenic cells affects cytosolic Ca2+-oscillations and cartilage formation
- PMID: 22132179
- PMCID: PMC3221679
- DOI: 10.1371/journal.pone.0027957
Switch of voltage-gated K+ channel expression in the plasma membrane of chondrogenic cells affects cytosolic Ca2+-oscillations and cartilage formation
Abstract
Background: Understanding the key elements of signaling of chondroprogenitor cells at the earliest steps of differentiation may substantially improve our opportunities for the application of mesenchymal stem cells in cartilage tissue engineering, which is a promising approach of regenerative therapy of joint diseases. Ion channels, membrane potential and Ca(2+)-signaling are important regulators of cell proliferation and differentiation. Our aim was to identify such plasma membrane ion channels involved in signaling during chondrogenesis, which may serve as specific molecular targets for influencing chondrogenic differentiation and ultimately cartilage formation.
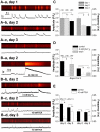
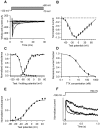
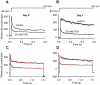
Methodology/principal findings: Using patch-clamp, RT-PCR and Western-blot experiments, we found that chondrogenic cells in primary micromass cell cultures obtained from embryonic chicken limb buds expressed voltage-gated Na(V)1.4, K(V)1.1, K(V)1.3 and K(V)4.1 channels, although K(V)1.3 was not detectable in the plasma membrane. Tetrodotoxin (TTX), the inhibitor of Na(V)1.4 channels, had no effect on cartilage formation. In contrast, presence of 20 mM of the K(+) channel blocker tetraethyl-ammonium (TEA) during the time-window of the final commitment of chondrogenic cells reduced K(V) currents (to 27±3% of control), cell proliferation (thymidine incorporation: to 39±4.4% of control), expression of cartilage-specific genes and consequently, cartilage formation (metachromasia: to 18.0±6.4% of control) and also depolarized the membrane potential (by 9.3±2.1 mV). High-frequency Ca(2+)-oscillations were also suppressed by 10 mM TEA (confocal microscopy: frequency to 8.5±2.6% of the control). Peak expression of TEA-sensitive K(V)1.1 in the plasma membrane overlapped with this period. Application of TEA to differentiated chondrocytes, mainly expressing the TEA-insensitive K(V)4.1 did not affect cartilage formation.
Conclusions/significance: These data demonstrate that the differentiation and proliferation of chondrogenic cells depend on rapid Ca(2+)-oscillations, which are modulated by K(V)-driven membrane potential changes. K(V)1.1 function seems especially critical during the final commitment period. We show the critical role of voltage-gated cation channels in the differentiation of non-excitable cells with potential therapeutic use.
Conflict of interest statement
Figures




References
-
- Centeno CJ, Busse D, Kisiday J, Keohan C, Freeman M, et al. Increased knee cartilage volume in degenerative joint disease using percutaneously implanted, autologous mesenchymal stem cells. Pain Physician. 2008;11:343–353. - PubMed
-
- Galle J, Bader A, Hepp P, Grill W, Fuchs B, et al. Mesenchymal stem cells in cartilage repair: state of the art and methods to monitor cell growth, differentiation and cartilage regeneration. Curr Med Chem. 2010;17:2274–2291. - PubMed
-
- Freyria AM, Mallein-Gerin F. Chondrocytes or adult stem cells for cartilage repair: The indisputable role of growth factors. Injury. 2011 In press: doi:10.1016/j.injury.2011.05.035. - PubMed
-
- Juretic N, Urzua U, Munroe DJ, Jaimovich E, Riveros N. Differential gene expression in skeletal muscle cells after membrane depolarization. J Cell Physiol. 2007;210:819–830. - PubMed
-
- Helmlinger G, Berk BC, Nerem RM. Pulsatile and steady flow-induced calcium oscillations in single cultured endothelial cells. J Vasc Res. 1996;33:360–369. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

