The glucuronyltransferase GlcAT-P is required for stretch growth of peripheral nerves in Drosophila
- PMID: 22132223
- PMCID: PMC3223219
- DOI: 10.1371/journal.pone.0028106
The glucuronyltransferase GlcAT-P is required for stretch growth of peripheral nerves in Drosophila
Abstract
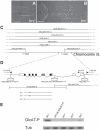
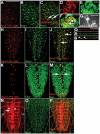
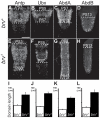
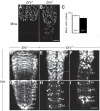
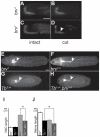
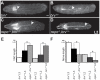
During development, the growth of the animal body is accompanied by a concomitant elongation of the peripheral nerves, which requires the elongation of integrated nerve fibers and the axons projecting therein. Although this process is of fundamental importance to almost all organisms of the animal kingdom, very little is known about the mechanisms regulating this process. Here, we describe the identification and characterization of novel mutant alleles of GlcAT-P, the Drosophila ortholog of the mammalian glucuronyltransferase b3gat1. GlcAT-P mutants reveal shorter larval peripheral nerves and an elongated ventral nerve cord (VNC). We show that GlcAT-P is expressed in a subset of neurons in the central brain hemispheres, in some motoneurons of the ventral nerve cord as well as in central and peripheral nerve glia. We demonstrate that in GlcAT-P mutants the VNC is under tension of shorter peripheral nerves suggesting that the VNC elongates as a consequence of tension imparted by retarded peripheral nerve growth during larval development. We also provide evidence that for growth of peripheral nerve fibers GlcAT-P is critically required in hemocytes; however, glial cells are also important in this process. The glial specific repo gene acts as a modifier of GlcAT-P and loss or reduction of repo function in a GlcAT-P mutant background enhances VNC elongation. We propose a model in which hemocytes are required for aspects of glial cell biology which in turn affects the elongation of peripheral nerves during larval development. Our data also identifies GlcAT-P as a first candidate gene involved in growth of integrated peripheral nerves and therefore establishes Drosophila as an amenable in-vivo model system to study this process at the cellular and molecular level in more detail.
Conflict of interest statement
Figures


Similar articles
-
Phosphatidylinositol 3-kinase and Akt nonautonomously promote perineurial glial growth in Drosophila peripheral nerves.J Neurosci. 2007 Jan 10;27(2):279-88. doi: 10.1523/JNEUROSCI.3370-06.2007. J Neurosci. 2007. PMID: 17215387 Free PMC article.
-
Developmental dynamics of peripheral glia in Drosophila melanogaster.Glia. 2000 Apr;30(2):122-33. doi: 10.1002/(sici)1098-1136(200004)30:2<122::aid-glia2>3.0.co;2-b. Glia. 2000. PMID: 10719354
-
Axonal ensheathment and septate junction formation in the peripheral nervous system of Drosophila.J Neurosci. 2006 Mar 22;26(12):3319-29. doi: 10.1523/JNEUROSCI.5383-05.2006. J Neurosci. 2006. PMID: 16554482 Free PMC article.
-
The specification of sensory neuron identity in Drosophila.Bioessays. 1993 May;15(5):293-8. doi: 10.1002/bies.950150502. Bioessays. 1993. PMID: 8343140 Review.
-
Enzyme assay of glucuronyltransferases for HNK-1 epitope (GlcAT-P, B3GAT1 and GlcAT-S, B3GAT2).2021 Sep 7 [updated 2022 Mar 14]. In: Nishihara S, Angata K, Aoki-Kinoshita KF, Hirabayashi J, editors. Glycoscience Protocols (GlycoPODv2) [Internet]. Saitama (JP): Japan Consortium for Glycobiology and Glycotechnology; 2021–. 2021 Sep 7 [updated 2022 Mar 14]. In: Nishihara S, Angata K, Aoki-Kinoshita KF, Hirabayashi J, editors. Glycoscience Protocols (GlycoPODv2) [Internet]. Saitama (JP): Japan Consortium for Glycobiology and Glycotechnology; 2021–. PMID: 37590727 Free Books & Documents. Review. No abstract available.
Cited by
-
Predetermined embryonic glial cells form the distinct glial sheaths of the Drosophila peripheral nervous system.Development. 2013 Sep;140(17):3657-68. doi: 10.1242/dev.093245. Epub 2013 Jul 31. Development. 2013. PMID: 23903191 Free PMC article.
-
Functional analysis of glycosylation using Drosophila melanogaster.Glycoconj J. 2020 Feb;37(1):1-14. doi: 10.1007/s10719-019-09892-0. Epub 2019 Nov 26. Glycoconj J. 2020. PMID: 31773367 Review.
-
Nutritionally driven differential gene expression leads to heterochronic brain development in honeybee castes.PLoS One. 2013 May 30;8(5):e64815. doi: 10.1371/journal.pone.0064815. Print 2013. PLoS One. 2013. PMID: 23738002 Free PMC article.
-
Sugar tags and tumorigenesis.Front Cell Dev Biol. 2015 Nov 4;3:69. doi: 10.3389/fcell.2015.00069. eCollection 2015. Front Cell Dev Biol. 2015. PMID: 26583080 Free PMC article. No abstract available.
-
Hemocyte Clusters Defined by scRNA-Seq in Bombyx mori: In Silico Analysis of Predicted Marker Genes and Implications for Potential Functional Roles.Front Immunol. 2022 Feb 25;13:852702. doi: 10.3389/fimmu.2022.852702. eCollection 2022. Front Immunol. 2022. PMID: 35281044 Free PMC article.
References
-
- Dickson BJ. Molecular mechanisms of axon guidance. Science. 2002;298:1959–1964. - PubMed
-
- Dent EW, Gertler FB. Cytoskeletal dynamics and transport in growth cone motility and axon guidance. Neuron. 2003;40:209–227. - PubMed
-
- Weiss P. Growth, Third Growth Symposium. pp; 1941. Nerve patterns: the mechanics of nerve growth. pp. 163–203.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

