Estimation of Abbreviated Cyclosporine A Area under the Concentration-Time Curve in Allogenic Stem Cell Transplantation after Oral Administration
- PMID: 22132303
- PMCID: PMC3205663
- DOI: 10.1155/2012/342701
Estimation of Abbreviated Cyclosporine A Area under the Concentration-Time Curve in Allogenic Stem Cell Transplantation after Oral Administration
Abstract
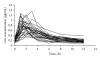
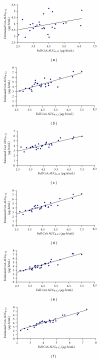
Measurements of Cyclosporine (CsA) systemic exposure permit its dose adjustment in allogenic stem cell transplantation recipients to prevent graft-versus-host disease. CsA LSSs were developed and validated from 60 ASCT patients via multiple linear regressions. All whole-blood samples were analyzed by fluorescence polarization immunoassay (FPIA-Axym). The 10 models that have used CsA concentrations at a single time point did not have a good fit with AUC(0-12) (R(2) < 0.90). C(2) and C(4) were the time points that correlated best with AUC(0-12 h), R(2) were respectively 0.848, and 0.897. The LSS equation with the best predictive performance (bias, precision and number of samples) utilized three sampling concentrations was AUC(0-12 h) = 0.607 + 1.569 × C(0.5) + 2.098 × C(2) + 3.603 × C(4) (R(2) = 0.943). Optimal LSSs equations which limited to those utilizing three timed concentrations taken within 4 hours post-dose developed from ASCT recipient's patients yielded a low bias <5% ranged from 1.27% to 2.68% and good precision <15% ranged from 9.60% and 11.02%. We propose an LSS model with equation AUC(0-12 h) = 0.82 + 2.766 × C(2) + 3.409 × C(4) for a practical reason. Bias and precision for this model are respectively 2.68% and 11.02%.
Figures
Similar articles
-
Limited sampling strategy for cyclosporine (Neoral) area under the curve monitoring in pediatric kidney transplant recipients.Pediatr Transplant. 2005 Oct;9(5):566-73. doi: 10.1111/j.1399-3046.2005.00339.x. Pediatr Transplant. 2005. PMID: 16176411
-
Prediction of area under the cyclosporine concentration versus time curve in children undergoing hematopoietic stem cell transplantation.Biol Blood Marrow Transplant. 2013 Mar;19(3):418-23. doi: 10.1016/j.bbmt.2012.10.031. Epub 2012 Nov 2. Biol Blood Marrow Transplant. 2013. PMID: 23128321
-
Population pharmacokinetics of ciclosporin in haematopoietic allogeneic stem cell transplantation with emphasis on limited sampling strategy.Br J Clin Pharmacol. 2012 Apr;73(4):553-63. doi: 10.1111/j.1365-2125.2011.04116.x. Br J Clin Pharmacol. 2012. PMID: 21988410 Free PMC article.
-
Limited sampling strategies for predicting area under the concentration-time curve of mycophenolic acid in islet transplant recipients.Ann Pharmacother. 2010 Jan;44(1):19-27. doi: 10.1345/aph.1M511. Epub 2009 Dec 8. Ann Pharmacother. 2010. PMID: 19996322
-
Limited sampling strategies drawn within 3 hours postdose poorly predict mycophenolic acid area-under-the-curve after enteric-coated mycophenolate sodium.Ther Drug Monit. 2009 Oct;31(5):585-91. doi: 10.1097/FTD.0b013e3181b8679a. Ther Drug Monit. 2009. PMID: 19704401 Clinical Trial.
Cited by
-
Therapeutic monitoring of pediatric renal transplant patients with conversion to generic cyclosporin.Int J Clin Pharm. 2014 Aug;36(4):779-86. doi: 10.1007/s11096-014-9959-0. Epub 2014 May 27. Int J Clin Pharm. 2014. PMID: 24861769
-
Bayesian approach for the estimation of cyclosporine area under the curve using limited sampling strategies in pediatric hematopoietic stem cell transplantation.Theor Biol Med Model. 2014 Sep 5;11:39. doi: 10.1186/1742-4682-11-39. Theor Biol Med Model. 2014. PMID: 25192585 Free PMC article.
-
A 16 Month Survey of Cyclosporine Utilization Evaluation in Allogeneic Hematopoietic Stem Cell Transplant Recipients.Iran J Pharm Res. 2016 Winter;15(1):331-9. Iran J Pharm Res. 2016. PMID: 27610174 Free PMC article.
-
Evaluation of Cyclosporine Pharmacokinetic, Monitoring, and Dosing Parameters for GVHD Prophylaxis in Hematopoietic Stem Cell Transplant (HSCT) Recipients.Iran J Pharm Res. 2019 Fall;18(Suppl1):302-314. doi: 10.22037/ijpr.2020.112111.13539. Iran J Pharm Res. 2019. PMID: 32802109 Free PMC article.
-
Population pharmacokinetics and Bayesian estimation of cyclosporine in a Tunisian population of hematopoietic stem cell transplant recipient.Eur J Clin Pharmacol. 2012 Nov;68(11):1517-24. doi: 10.1007/s00228-012-1275-9. Epub 2012 Apr 15. Eur J Clin Pharmacol. 2012. PMID: 22527344
References
-
- Duncan N, Craddock C. Optimizing the use of cyclosporin in allogeneic stem cell transplantation. Bone Marrow Transplantation. 2006;38(3):169–174. - PubMed
-
- Schultz KR, Nevill TJ, Balshaw RF, et al. Effect of gastrointestinal inflammation and age on the pharmacokinetics of oral microemulsion cyclosporin A in the first month after bone marrow transplantation. Bone Marrow Transplantation. 2000;26(5):545–551. - PubMed
-
- Ferrara JLM, Cooke KR, Teshima T. The pathophysiology of acute graft-versus-host disease. International Journal of Hematology. 2003;78(3):181–187. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

