Dystrophin deficiency exacerbates skeletal muscle pathology in dysferlin-null mice
- PMID: 22132688
- PMCID: PMC3287108
- DOI: 10.1186/2044-5040-1-35
Dystrophin deficiency exacerbates skeletal muscle pathology in dysferlin-null mice
Abstract
Background: Mutations in the genes coding for either dystrophin or dysferlin cause distinct forms of muscular dystrophy. Dystrophin links the cytoskeleton to the sarcolemma through direct interaction with β-dystroglycan. This link extends to the extracellular matrix by β-dystroglycan's interaction with α-dystroglycan, which binds extracellular matrix proteins, including laminin α2, agrin and perlecan, that possess laminin globular domains. The absence of dystrophin disrupts this link, leading to compromised muscle sarcolemmal integrity. Dysferlin, on the other hand, plays an important role in the Ca2+-dependent membrane repair of damaged sarcolemma in skeletal muscle. Because dysferlin and dystrophin play different roles in maintaining muscle cell integrity, we hypothesized that disrupting sarcolemmal integrity with dystrophin deficiency would exacerbate the pathology in dysferlin-null mice and allow further characterization of the role of dysferlin in skeletal muscle.
Methods: To test our hypothesis, we generated dystrophin/dysferlin double-knockout (DKO) mice by breeding mdx mice with dysferlin-null mice and analyzed the effects of a combined deficiency of dysferlin and dystrophin on muscle pathology and sarcolemmal integrity.
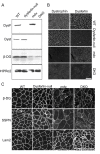
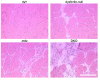
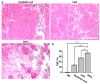
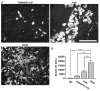
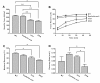
Results: The DKO mice exhibited more severe muscle pathology than either mdx mice or dysferlin-null mice, and, importantly, the onset of the muscle pathology occurred much earlier than it did in dysferlin-deficient mice. The DKO mice showed muscle pathology of various skeletal muscles, including the mandible muscles, as well as a greater number of regenerating muscle fibers, higher serum creatine kinase levels and elevated Evans blue dye uptake into skeletal muscles. Lengthening contractions caused similar force deficits, regardless of dysferlin expression. However, the rate of force recovery within 45 minutes following lengthening contractions was hampered in DKO muscles compared to mdx muscles or dysferlin-null muscles, suggesting that dysferlin is required for the initial recovery from lengthening contraction-induced muscle injury of the dystrophin-glycoprotein complex-compromised muscles.
Conclusions: The results of our study suggest that dysferlin-mediated membrane repair helps to limit the dystrophic changes in dystrophin-deficient skeletal muscle. Dystrophin deficiency unmasks the function of dysferlin in membrane repair during lengthening contractions. Dystrophin/dysferlin-deficient mice provide a very useful model with which to evaluate the effectiveness of therapies designed to treat dysferlin deficiency.
Figures
References
-
- Han R, Kanagawa M, Yoshida-Moriguchi T, Rader EP, Ng RA, Michele DE, Muirhead DE, Kunz S, Moore SA, Iannaccone ST, Miyake K, McNeil PL, Mayer U, Oldstone MB, Faulkner JA, Campbell KP. Basal lamina strengthens cell membrane integrity via the laminin G domain-binding motif of α-dystroglycan. Proc Natl Acad Sci USA. 2009;106:12573–12579. doi: 10.1073/pnas.0906545106. - DOI - PMC - PubMed
-
- Clarke MS, Khakee R, McNeil PL. Loss of cytoplasmic basic fibroblast growth factor from physiologically wounded myofibers of normal and dystrophic muscle. J Cell Sci. 1993;106:121–133. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

