Translational Mini-Review Series on B cell subsets in disease. Reconstitution after haematopoietic stem cell transplantation - revelation of B cell developmental pathways and lineage phenotypes
- PMID: 22132880
- PMCID: PMC3248082
- DOI: 10.1111/j.1365-2249.2011.04469.x
Translational Mini-Review Series on B cell subsets in disease. Reconstitution after haematopoietic stem cell transplantation - revelation of B cell developmental pathways and lineage phenotypes
Abstract
Haematopoietic stem cell transplantation (HSCT) is an immunological treatment that has been used for more than 40 years to cure a variety of diseases. The procedure is associated with serious side effects, due to the severe impairment of the immune system induced by the treatment. After a conditioning regimen with high-dose chemotherapy, sometimes in combination with total body irradiation, haematopoietic stem cells are transferred from a donor, allowing a donor-derived blood system to form. Here, we discuss the current knowledge of humoral problems and B cell development after HSCT, and relate these to the current understanding of human peripheral B cell development. We describe how these studies have aided the identification of subsets of transitional B cells and also a robust memory B cell phenotype.
© 2011 The Authors. Clinical and Experimental Immunology © 2011 British Society for Immunology.
Figures


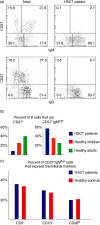
References
-
- Ek T, Mellander L, Andersson B, Abrahamsson J. Immune reconstitution after childhood acute lymphoblastic leukemia is most severely affected in the high risk group. Pediatr Blood Cancer. 2005;44:461–8. - PubMed
-
- Torelli GF, Lucarelli B, Iori AP, et al. The immune reconstitution after an allogeneic stem cell transplant correlates with the risk of graft-versus-host disease and cytomegalovirus infection. Leuk Res. 2011;35:1124–6. - PubMed
-
- Storek J, Geddes M, Khan F, et al. Reconstitution of the immune system after hematopoietic stem cell transplantation in humans. Semin Immunopathol. 2008;30:425–37. - PubMed
-
- Sahdev I, O'Reilly R, Black P, Heller G, Hoffmann M. Interleukin-1 production following T-cell-depleted and unmodified marrow grafts. Pediatr Hematol Oncol. 1996;13:55–67. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

