Essential role of the adaptor protein Nck1 in Jurkat T cell activation and function
- PMID: 22132889
- PMCID: PMC3248091
- DOI: 10.1111/j.1365-2249.2011.04494.x
Essential role of the adaptor protein Nck1 in Jurkat T cell activation and function
Abstract
The non-catalytic region of tyrosine kinase (Nck) is proposed to play an essential role in T cell activation. However, evidence based on functional and biochemical studies has brought into question the critical function of Nck. Therefore, the aim of the present work was to investigate the role of Nck in T cell activation. To study this, the human Jurkat T cell line was used as a model for human T lymphocytes. The short interfering (si) RNA targeting Nck1 gene was used with electroporation to knock-down Nck1 protein expression in Jurkat T cells. Primary human CD4 T cells were also transfected with the siRNA of Nck1. The results showed that decreased Nck1 protein expression did not affect the apoptosis of the transfected Jurkat T cells compared with control siRNA-transfected cells and non-transfected cells. Upon CD3ε/CD28 stimulation, knock-down of Nck1 in Jurkat T cells caused a decrease in CD69 expression and in interleukin (IL)-2 secretion. Similarly, knock-down of Nck1 in primary CD4 T cells also caused decreased CD69 expression. However, no significant alterations of CD69 and IL-2 expression were found upon phytohaemagglutinin (PHA)/phorbol myristate acetate (PMA) stimulation. Knock-down of Nck1 had no effect on the proliferation of Jurkat T cells stimulated with either PHA or anti-T cell receptor (TCR) monoclonal antibody (C305). The reduced Nck1 expression in Jurkat cells was also associated with a reduced phosphorylation of extracellular regulated kinase (Erk)1 and Erk2 proteins upon CD3ε/CD28 stimulation. In conclusion, the decreased Nck1 protein in Jurkat T cells resulted in an impairment of TCR-CD3-mediated activation involving a defective Erk phosphorylation pathway.
© 2011 The Authors. Clinical and Experimental Immunology © 2011 British Society for Immunology.
Figures
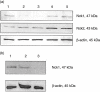
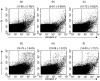
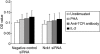
 ), anti-T cell receptor (TCR) antibody (
), anti-T cell receptor (TCR) antibody ( ) or interleukin (IL)-2 (
) or interleukin (IL)-2 ( ) for 24 h and proliferation measured using 5-bromo-2′-deoxyuridine (BrdU). Values are presented as mean ± standard deviation from three experiments. OD: optical density read at 450 nm.
) for 24 h and proliferation measured using 5-bromo-2′-deoxyuridine (BrdU). Values are presented as mean ± standard deviation from three experiments. OD: optical density read at 450 nm.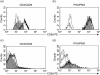
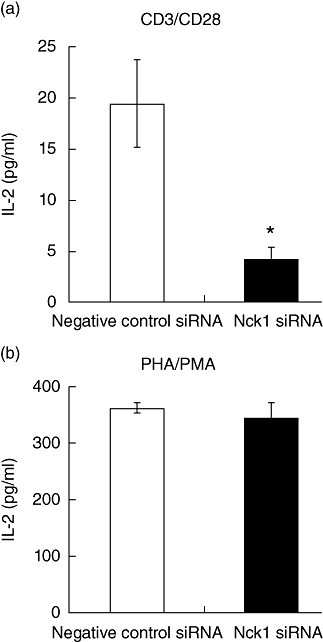
 ) Jurkat T cells were stimulated with anti-CD3ε/anti-CD28 antibodies (a) or phytohaemagglutinin (PHA)/phorbol myristate acetate (PMA) (b) for 24 h. Results represent mean ± standard deviation of three experiments. *P < 0·05.
) Jurkat T cells were stimulated with anti-CD3ε/anti-CD28 antibodies (a) or phytohaemagglutinin (PHA)/phorbol myristate acetate (PMA) (b) for 24 h. Results represent mean ± standard deviation of three experiments. *P < 0·05.
Similar articles
-
Non-overlapping functions of Nck1 and Nck2 adaptor proteins in T cell activation.Cell Commun Signal. 2014 Mar 26;12:21. doi: 10.1186/1478-811X-12-21. Cell Commun Signal. 2014. PMID: 24670066 Free PMC article.
-
The adaptor protein NTAL enhances proximal signaling and potentiates corticosteroid-induced apoptosis in T-ALL.Exp Hematol. 2012 May;40(5):379-85. doi: 10.1016/j.exphem.2012.01.011. Epub 2012 Jan 21. Exp Hematol. 2012. PMID: 22269118
-
Decreased CD154 expression by neonatal CD4+ T cells is due to limitations in both proximal and distal events of T cell activation.Int Immunol. 2003 Dec;15(12):1461-72. doi: 10.1093/intimm/dxg145. Int Immunol. 2003. PMID: 14645155
-
Actin polymerization regulates recruitment of Nck to CD3ε upon T-cell receptor triggering.Immunology. 2020 Mar;159(3):298-308. doi: 10.1111/imm.13146. Epub 2019 Nov 26. Immunology. 2020. PMID: 31674657 Free PMC article.
-
PRR7 is a transmembrane adaptor protein expressed in activated T cells involved in regulation of T cell receptor signaling and apoptosis.J Biol Chem. 2011 Jun 3;286(22):19617-29. doi: 10.1074/jbc.M110.175117. Epub 2011 Apr 1. J Biol Chem. 2011. PMID: 21460222 Free PMC article.
Cited by
-
Characterization of an air-liquid interface primary human vaginal epithelium to study Ebola virus infection and testing of antivirals.Antiviral Res. 2023 Mar;211:105551. doi: 10.1016/j.antiviral.2023.105551. Epub 2023 Jan 31. Antiviral Res. 2023. PMID: 36731656 Free PMC article.
-
Downregulation of Nck1 After Spinal Cord Injury in Adult Rats.Balkan Med J. 2022 Jan 25;39(1):39-47. doi: 10.5152/balkanmedj.2021.06114. Epub 2021 Dec 20. Balkan Med J. 2022. PMID: 34928232 Free PMC article.
-
Non-overlapping functions of Nck1 and Nck2 adaptor proteins in T cell activation.Cell Commun Signal. 2014 Mar 26;12:21. doi: 10.1186/1478-811X-12-21. Cell Commun Signal. 2014. PMID: 24670066 Free PMC article.
-
Ovalbumin Antigen-Specific Activation of Human T Cell Receptor Closely Resembles Soluble Antibody Stimulation as Revealed by BOOST Phosphotyrosine Proteomics.J Proteome Res. 2021 Jun 4;20(6):3330-3344. doi: 10.1021/acs.jproteome.1c00239. Epub 2021 May 21. J Proteome Res. 2021. PMID: 34018748 Free PMC article.
-
Nck recruitment to the TCR required for ZAP70 activation during thymic development.J Immunol. 2013 Feb 1;190(3):1103-12. doi: 10.4049/jimmunol.1202055. Epub 2012 Dec 24. J Immunol. 2013. PMID: 23267019 Free PMC article.
References
-
- Park D. Cloning, sequencing, and overexpression of SH2/SH3 adaptor protein Nck from mouse thymus. Mol Cells. 1997;7:231–36. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

