Health-related quality of life and treatment satisfaction in the Sensor-Augmented Pump Therapy for A1C Reduction 3 (STAR 3) trial
- PMID: 22133037
- PMCID: PMC4845679
- DOI: 10.1089/dia.2011.0162
Health-related quality of life and treatment satisfaction in the Sensor-Augmented Pump Therapy for A1C Reduction 3 (STAR 3) trial
Abstract
Objective: This study assessed health-related quality of life (HRQOL) and treatment satisfaction in sensor-augmented pump therapy (SAPT) compared with optimal conventional therapy-multiple daily injection (MDI) therapy with self-monitoring of blood glucose (SMBG)-in adults and children with type 1 diabetes and children's caregivers. Patient acceptance of new therapies is essential to their adoption and effective use.
Research design and methods: STAR 3, a randomized 12-month clinical trial, compared SAPT with MDI+SMBG in 485 adult and pediatric patients. Within- and between-treatment arm changes in generic HRQOL, diabetes-specific HRQOL (fear of hypoglycemia), and treatment satisfaction were assessed (significance criterion P<0.01).
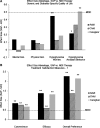
Results: In adults, children, and caregivers, there were no significant between-arm changes in generic HRQOL: SF-36 Physical Component Summary and Mental Component Summary scores in adults and the PedsQL Physical Health Summary and Psychosocial Health Summary scores in children or caregivers. Diabetes-specific HRQOL (Hypoglycemia Fear Survey Worry and Behavior subscale scores) improved more in SAPT than in MDI adults. Hypoglycemia Behavior scores improved more in SAPT caregivers. Key treatment satisfaction measures (Insulin Delivery System Rating Questionnaire measures of Convenience, Efficacy, and Overall Preference) improved more in SAPT adults, children, and caregivers (all P<0.001); all exceeded the criterion for minimal detectable difference.
Conclusions: In the first-ever large-scale study of SAPT compared with optimal conventional therapy, SAPT had significant advantages for hypoglycemia fear in adults and caregivers and for treatment satisfaction in adults, children, and caregivers.
Figures

References
-
- The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. Diabetes Control and Complications Trial Research Group. N Engl J Med. 1993;329:977–986. - PubMed
-
- Nathan DM. Cleary PA. Backlund JY. Genuth SM. Lachin JM. Orchard TJ. Raskin P. Zinman B Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDICT) Study Research Group. Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med. 2005;353:2643–2653. - PMC - PubMed
-
- Misso ML. Egberts KJ. Page M. O'Connor D. Shaw J. Continuous subcutaneous insulin infusion (CSII) versus multiple insulin injections for type 1 diabetes mellitus. Cochrane Database Syst Rev. 2010;1:CD005103. - PubMed
-
- The Juvenile Diabetes Research Foundation Continuous Glucose Monitoring Study Group. Continuous glucose monitoring and intensive treatment of type 1 diabetes. N Engl J Med. 2008;359:1464–1476. - PubMed
-
- Hirsch IB. Abelseth J. Bode BW. Fischer J. Kaufman FR. Mastrototaro J. Parkin CG. Wolpert HA. Buckingham BA. Sensor-augmented insulin pump therapy: results of the first randomized treat-to-target study. Diabetes Technol Ther. 2008;10:377–383. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

