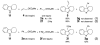
Palladium-catalyzed C3-benzylation of indoles
- PMID: 22133348
- PMCID: PMC3383063
- DOI: 10.1021/ja2095393
Palladium-catalyzed C3-benzylation of indoles
Abstract
A general method for regioselective C3-benzylation of indoles has been developed. Various 3-substituted indoles and benzyl methyl carbonates with different electronic properties react under mild conditions to afford a diverse range of 3-benzylindolenine products in good yields.
© 2011 American Chemical Society
Figures
Similar articles
-
Palladium-catalyzed decarboxylative allylation and benzylation of N-alloc and N-Cbz indoles.Org Lett. 2013 Mar 1;15(5):1140-3. doi: 10.1021/ol400334u. Epub 2013 Feb 21. Org Lett. 2013. PMID: 23427905 Free PMC article.
-
Palladium-catalyzed direct benzylation of azoles with benzyl carbonates.Org Lett. 2010 Mar 19;12(6):1360-3. doi: 10.1021/ol1002576. Org Lett. 2010. PMID: 20180547
-
Rapid access to spirocyclized indolenines via palladium-catalyzed cascade reactions of tryptamine derivatives and propargyl carbonate.Org Lett. 2014 Jul 3;16(13):3480-3. doi: 10.1021/ol501409a. Epub 2014 Jun 25. Org Lett. 2014. PMID: 24964382 Free PMC article.
-
Pd(0)-catalyzed alkenylation and allylic dearomatization reactions between nucleophile-bearing indoles and propargyl carbonate.Org Lett. 2014 Aug 1;16(15):3919-21. doi: 10.1021/ol501704q. Epub 2014 Jul 3. Org Lett. 2014. PMID: 24992703
-
Development of palladium-catalyzed transformations using propargylic compounds.Chem Pharm Bull (Tokyo). 2012;60(3):285-99. doi: 10.1248/cpb.60.285. Chem Pharm Bull (Tokyo). 2012. PMID: 22382407 Review.
Cited by
-
Palladium-catalyzed substitution of (coumarinyl)methyl acetates with C-, N-, and S-nucleophiles.Beilstein J Org Chem. 2012;8:1200-7. doi: 10.3762/bjoc.8.133. Epub 2012 Jul 27. Beilstein J Org Chem. 2012. PMID: 23019448 Free PMC article.
-
An intramolecular cascade cyclization of 2-aryl indoles: efficient methods for the construction of 2,3-functionalized indolines and 3-indolinones.Org Biomol Chem. 2014 Jun 14;12(22):3567-71. doi: 10.1039/c4ob00511b. Epub 2014 May 1. Org Biomol Chem. 2014. PMID: 24788461 Free PMC article.
-
A Copper-Catalyzed Arylation of Tryptamines for the Direct Synthesis of Aryl Pyrroloindolines.Chem Sci. 2012 Nov;3(11):3170-3174. doi: 10.1039/C2SC20914D. Chem Sci. 2012. PMID: 23105962 Free PMC article.
-
Pd-Catalyzed Alkene Carboheteroarylation Reactions for the Synthesis of 3-Cyclopentylindole Derivatives.J Org Chem. 2018 Nov 2;83(21):13568-13573. doi: 10.1021/acs.joc.8b02165. Epub 2018 Oct 10. J Org Chem. 2018. PMID: 30351050 Free PMC article.
-
Rapid assembly of functionalised spirocyclic indolines by palladium-catalysed dearomatising diallylation of indoles with allyl acetate.Chemistry. 2014 Oct 6;20(41):13375-81. doi: 10.1002/chem.201403940. Epub 2014 Aug 29. Chemistry. 2014. PMID: 25171550 Free PMC article.
References
-
- Cacchi S, Fabrizi G. Chem. Rev. 2005;105:2873. - PubMed
- Chem. Rev. 2011;111:PR215. Update 1: - PubMed
- Joucla L, Djakovitch L. Adv. Synth. Catal. 2009;351:673.
- Bandini M, Eichholzer A. Angew. Chem. Int. Ed. 2009;48:9608. - PubMed
- Bartoli G, Bencivenni G, Dalpozzo R. Chem. Soc. Rev. 2010;39:4449. - PubMed
-
-
For selected examples of Pd-catalyzed allylation reaction of 3-unsubstituted indoles: Bandini M, Melloni A, Umani-Ronchi A. Org. Lett. 2004;6:3199. Kimura M, Futamata M, Mukai R, Tamaru Y. J. Am. Chem. Soc. 2005;127:4592. Bandini M, Melloni A, Piccinelli F, Sinisi R, Tommasi S, Umani-Ronchi A. J. Am. Chem. Soc. 2006;128:1424. 3-Substituted indoles: Trost BM, Quancard J. J. Am. Chem. Soc. 2006;128:6314. Kagawa N, Malerich JP, Rawal VH. Org. Lett. 2008;10:2381.
-
-
-
For examples of alkylation of indolyl anion using alkyl halide: Hoshino T. Liebigs Ann. Chem. 1933;500:35. Nakazaki M. Bull. Chem. Soc. Jpn. 1959;32:838. Reactions between indoles and o-halomethyl phenol/aniline: Spande TF, Wilchek M, Witkop B. J. Am. Chem. Soc. 1968;90:3256. May JA, Zeidan RK, Stoltz BM. Tetrahedron Lett. 2003;44:1203.
-
-
-
For selected examples using indolenines as intermediates in syntheses: Robinson R, Suginome H. J. Chem. Soc. 1932:298. Woodward RB, Cava MP, Ollis WD, Hunger A, Daeniker HU, Schenker K. J. Am. Chem. Soc. 1954;76:4749. Stork G, Dolfini JE. J. Am. Chem. Soc. 1963;85:2872. He F, Bo Y, Altom JD, Corey EJ. J. Am. Chem. Soc. 1999;121:6771. Kozmin SA, Iwama T, Huang Y, Rawal VH. J. Am. Chem. Soc. 2002;124:4628.
-
-
- Numata A, Takahashi C, Ito Y, Takada T, Kawai K, Usami Y, Matsumura E, Imachi M, Ito T, Hasegawa T. Tetrahedron Lett. 1993;34:2355.
- Verbitski SM, Mayne CL, Davis RA, Concepcion GP, Ireland CM. J. Org. Chem. 2002;67:7124. - PubMed
- Yu S, Qin D, Shangary S, Chen J, Wang G, Ding K, McEachern D, Qiu S, Nikolovska-Coleska Z, Miller R, Kang S, Yang D, Wang S. J. Med. Chem. 2009;52:7970. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous