Long-term effects of intravenous iloprost in patients with idiopathic pulmonary arterial hypertension deteriorating on non-parenteral therapy
- PMID: 22133492
- PMCID: PMC3247176
- DOI: 10.1186/1471-2466-11-56
Long-term effects of intravenous iloprost in patients with idiopathic pulmonary arterial hypertension deteriorating on non-parenteral therapy
Abstract
Background: The majority of patients with idiopathic pulmonary arterial hypertension (IPAH) in functional classes II and III are currently being treated with non-parenteral therapies, including endothelin receptor antagonists (ERA), phosphodiesterase (PDE)-5 inhibitors, inhaled iloprost or combinations of these substances. If these treatments fail, current guidelines recommend the addition of parenteral prostanoid therapy. There is, however, limited evidence for the efficacy of parenteral prostanoids when added to combinations of non-parenteral therapies.
Methods: In this retrospective, multicentre study we collected data from consecutive IPAH patients receiving intravenous iloprost in addition to optimized non-parenteral therapy between Jan 2002 and Dec 2009. Analyses included 6 min walk distance (6MWD), functional class, need for transplantation, and survival.
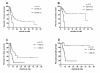
Results: During the observation period, 50 patients were treated with intravenous iloprost in addition to non-parenteral therapy; 44% of the patients were on dual combination therapy and 52% on triple combination. Three months after initiation of iloprost, functional class had improved in 24% of the patients and the median 6MWD had increased from 289 m to 298 m (n.s.). During the observation period, 22 patients (44%) died and 14 (28%) underwent lung transplantation. The probabilities of LuTx-free survival at 1, 3 and 5 years following iloprost initiation were 38%, 17% and 17%, respectively. A 6MWD < 300 m and persistent functional class IV at 3 months after initiation of intravenous iloprost were predictors of an adverse outcome.
Conclusion: In essence, late initiation of intravenous iloprost in IPAH patients who previously failed to respond to non-parenteral therapies appears to be of limited efficacy in the majority patients. Alternative therapeutic options are currently not available, underlying the need for the development of new drugs.
Figures

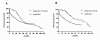
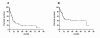
Similar articles
-
Oral sildenafil as long-term adjunct therapy to inhaled iloprost in severe pulmonary arterial hypertension.J Am Coll Cardiol. 2003 Jul 2;42(1):158-64. doi: 10.1016/s0735-1097(03)00555-2. J Am Coll Cardiol. 2003. PMID: 12849677 Clinical Trial.
-
Combining inhaled iloprost with bosentan in patients with idiopathic pulmonary arterial hypertension.Eur Respir J. 2006 Oct;28(4):691-4. doi: 10.1183/09031936.06.00057906. Eur Respir J. 2006. PMID: 17012628 Clinical Trial.
-
Intravenous iloprost for treatment failure of aerosolised iloprost in pulmonary arterial hypertension.Eur Respir J. 2002 Aug;20(2):339-43. doi: 10.1183/09031936.02.02462001. Eur Respir J. 2002. PMID: 12212965
-
Review of inhaled iloprost for the control of pulmonary artery hypertension in children.Vasc Health Risk Manag. 2009;5(1):325-31. doi: 10.2147/vhrm.s3222. Epub 2009 Apr 8. Vasc Health Risk Manag. 2009. PMID: 19436672 Free PMC article. Review.
-
[Drug combination treatment for pulmonary arterial hypertension].Dtsch Med Wochenschr. 2006 Dec 8;131(49 Suppl 9):S330-3. doi: 10.1055/s-2006-957205. Dtsch Med Wochenschr. 2006. PMID: 17139600 Review. German.
Cited by
-
Central venous catheter-related blood stream infections in patients receiving intravenous iloprost for pulmonary hypertension.Eur J Clin Microbiol Infect Dis. 2013 Jul;32(7):883-9. doi: 10.1007/s10096-013-1822-z. Epub 2013 Feb 7. Eur J Clin Microbiol Infect Dis. 2013. PMID: 23388830
-
How to Solve the Conundrum of Heparin-Induced Thrombocytopenia during Cardiopulmonary Bypass.J Clin Med. 2023 Jan 18;12(3):786. doi: 10.3390/jcm12030786. J Clin Med. 2023. PMID: 36769435 Free PMC article. Review.
-
An update on medical therapy for pulmonary arterial hypertension.Curr Hypertens Rep. 2013 Dec;15(6):614-22. doi: 10.1007/s11906-013-0394-8. Curr Hypertens Rep. 2013. PMID: 24122306 Review.
-
Central line replacement following infection does not improve reinfection rates in pediatric pulmonary hypertension patients receiving intravenous prostanoid therapy.Pulm Circ. 2018 Jan-Mar;8(1):2045893218754886. doi: 10.1177/2045893218754886. Epub 2018 Jan 8. Pulm Circ. 2018. PMID: 29309237 Free PMC article.
-
Incidence of Bloodstream Infection in Patients with Pulmonary Hypertension under Intravenous Epoprostenol or Iloprost-A Multicentre, Retrospective Study.Int J Mol Sci. 2023 Mar 29;24(7):6434. doi: 10.3390/ijms24076434. Int J Mol Sci. 2023. PMID: 37047407 Free PMC article.
References
-
- Barst R, Rubin L, Long W, McGoon M, Rich S, Badesch D, Groves B, Tapson V, Bourge R, Brundage B. A comparison of continuous intravenous epoprostenol (prostacyclin) with conventional therapy for primary pulmonary hypertension. The Primary Pulmonary Hypertension Study Group. N Engl J Med. 1996;334(5):296–302. doi: 10.1056/NEJM199602013340504. - DOI - PubMed
-
- Ewert R, Opitz C, Wensel R, Winkler J, Halank M, Felix S. Continuous intravenous iloprost to revert treatment failure of first-line inhaled iloprost therapy in patients with idiopathic pulmonary arterial hypertension. Clin Res Cardiol. 2007;96(4):211–217. doi: 10.1007/s00392-007-0490-3. - DOI - PubMed
-
- Safdar Z. Treatment of pulmonary arterial hypertension: The role of prostacyclin and prostaglandin analogs. Respir Med. 2011. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

