Brief exposure to progesterone during a critical neonatal window prevents uterine gland formation in mice
- PMID: 22133692
- PMCID: PMC3316263
- DOI: 10.1095/biolreprod.111.097188
Brief exposure to progesterone during a critical neonatal window prevents uterine gland formation in mice
Abstract
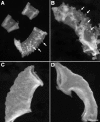
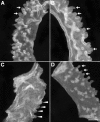
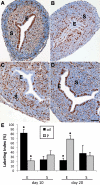
Uterine gland development (adenogenesis) in mice begins on Postnatal Day (PND) 5 and is completed in adulthood. Adenogenesis depends on estrogen receptor 1, and progesterone (P4) inhibits mitogenic effects of estrogen on uterine epithelium. This progestin-induced effect has been used to inhibit uterine gland development; progestin treatment of ewes for 8 wk from birth has produced infertile adults lacking uterine glands. The goals of the present study were to determine if a window of susceptibility to P4-mediated inhibition of uterine gland development exists in mice and whether early P4 treatment abolishes adenogenesis and fertility. Mice were injected daily with P4 (40 μg/g) or vehicle during various postnatal windows. Adenogenesis, cell proliferation, and expression of key morphoregulatory transcripts and proteins were examined in uteri at PNDs 10 and 20. Additionally, adenogenesis was assessed in isolated uterine epithelium. Treatment during PNDs 3-9, 5-9, or 3-7 abolished adenogenesis at PND 10, whereas treatments during PNDs 3-5 and 7-9 did not. Critically, mice treated during PNDs 3-9 lacked glands in adulthood, indicating that adenogenesis did not resume after this treatment. However, glands were present by PND 20 and later following treatment during PNDs 5-9 or 3-7, whereas treatment during PNDs 10-16 produced partial inhibition of adenogenesis at PND 20 and later. Epithelial proliferation at PND 10 was low following P4 treatment (PNDs 3-9) but exceeded that in controls at PND 20, indicating a rebound of epithelial proliferation following treatment. Messenger RNA for Wnt, Fzd, and Hox genes was altered by neonatal P4 treatment. All groups cycled during adulthood. Mice treated with P4 during PNDs 3-9, but not during other developmental windows, showed minimal fertility in adulthood. In summary, brief P4 treatment (7 days) during a critical neonatal window (PNDs 3-9) transiently inhibited epithelial proliferation but totally and permanently blocked adenogenesis and adult fertility. This resulted in permanent loss of uterine glands and, essentially, total infertility during adulthood. The narrow window for inhibition of adenogenesis identified here may have implications for development of this methodology as a contraceptive strategy for animals.
Figures

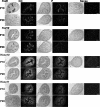
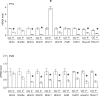

Similar articles
-
Progesterone inhibits uterine gland development in the neonatal mouse uterus.Biol Reprod. 2012 May 10;86(5):146, 1-9. doi: 10.1095/biolreprod.111.097089. Print 2012 May. Biol Reprod. 2012. PMID: 22238285 Free PMC article.
-
Uterine glands: development, function and experimental model systems.Mol Hum Reprod. 2013 Sep;19(9):547-58. doi: 10.1093/molehr/gat031. Epub 2013 Apr 25. Mol Hum Reprod. 2013. PMID: 23619340 Free PMC article. Review.
-
Uterine gland development begins postnatally and is accompanied by estrogen and progesterone receptor expression in the dog.Theriogenology. 2012 Nov;78(8):1787-95. doi: 10.1016/j.theriogenology.2012.05.028. Epub 2012 Sep 7. Theriogenology. 2012. PMID: 22959316
-
Neonatal ovine uterine development involves alterations in expression of receptors for estrogen, progesterone, and prolactin.Biol Reprod. 2000 Oct;63(4):1192-204. doi: 10.1095/biolreprod63.4.1192. Biol Reprod. 2000. PMID: 10993845
-
Uterine and placental factors regulating conceptus growth in domestic animals.J Anim Sci. 2004;82 E-Suppl:E4-13. doi: 10.2527/2004.8213_supplE4x. J Anim Sci. 2004. PMID: 15471813 Review.
Cited by
-
Chronic Estrus Disrupts Uterine Gland Development and Homeostasis.Endocrinology. 2022 Mar 1;163(3):bqac011. doi: 10.1210/endocr/bqac011. Endocrinology. 2022. PMID: 35134138 Free PMC article.
-
Volumetric imaging of the developing prepubertal mouse uterine epithelium using light sheet microscopy.Mol Reprod Dev. 2018 May;85(5):397-405. doi: 10.1002/mrd.22973. Epub 2018 May 3. Mol Reprod Dev. 2018. PMID: 29543367 Free PMC article.
-
Uterine Patterning, Endometrial Gland Development, and Implantation Failure in Mice Exposed Neonatally to Genistein.Environ Health Perspect. 2020 Mar;128(3):37001. doi: 10.1289/EHP6336. Epub 2020 Mar 18. Environ Health Perspect. 2020. PMID: 32186404 Free PMC article.
-
The glands have it.Biol Reprod. 2013 Apr 11;88(4):94. doi: 10.1095/biolreprod.113.108944. Print 2013 Apr. Biol Reprod. 2013. PMID: 23486911 Free PMC article.
-
Molecular Signaling Regulating Endometrium-Blastocyst Crosstalk.Int J Mol Sci. 2019 Dec 18;21(1):23. doi: 10.3390/ijms21010023. Int J Mol Sci. 2019. PMID: 31861484 Free PMC article. Review.
References
-
- Gray CA, Bartol FF, Tarleton BJ, Wiley AA, Johnson GA, Bazer FW, Spencer TE. Developmental biology of uterine glands. Biol Reprod 2001; 65: 1311 1323 - PubMed
-
- Spencer TE, Hayashi K, Hu J, Carpenter KD. Comparative developmental biology of the mammalian uterus. Curr Top Dev Biol 2005; 68: 85 122 - PubMed
-
- Ogasawara Y, Okamoto S, Kitamura Y, Matsumoto K. Proliferative pattern of uterine cells from birth to adulthood in intact, neonatally castrated, and/or adrenalectomized mice, assayed by incorporation of [125I]iododeoxyuridine. Endocrinology 1983; 113: 582 587 - PubMed
-
- Bigsby RM, Cunha GR. Estrogen stimulation of deoxyribonucleic acid synthesis in uterine epithelial cells which lack estrogen receptors. Endocrinology 1986; 119: 390 396 - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

