Phase II trial of vorinostat and gemtuzumab ozogamicin as induction and post-remission therapy in older adults with previously untreated acute myeloid leukemia
- PMID: 22133771
- PMCID: PMC3342977
- DOI: 10.3324/haematol.2011.055822
Phase II trial of vorinostat and gemtuzumab ozogamicin as induction and post-remission therapy in older adults with previously untreated acute myeloid leukemia
Abstract
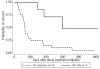
Histone deacetylase inhibitors such as vorinostat enhance gemtuzumab ozogamicin efficacy in vitro. We, therefore, investigated vorinostat+gemtuzumab ozogamicin for adults aged 60 years and over with untreated acute myeloid leukemia. We stratified patients into 2 groups (group 1: patients aged ≥ 70 years and performance status 2-3; group 2: aged 60-69 years with performance status 0-3 or aged ≥ 70 years and performance status 0-1). Responses were monitored separately in group 2 patients with normal or favorable cytogenetics (group 2A) and other cytogenetics (group 2B). Among 31 patients, 6 (19.4%) achieved complete remission, and one (3.2%) achieved complete remission with incomplete platelet recovery; these patients had a higher median overall survival than non-responders (553 vs. 131 days, P = 0.0026). Response rates were: group 1, one of 10 (10.0%); group 2A, 6 of 13 (46.2%); and group 2B, none of 8 (0%). These data indicate that vorinostat+gemtuzumab ozogamicin has activity that is mostly confined to patients with normal karyotype disease. ClinicalTrial.gov: NCT00673153.
Figures
Similar articles
-
Gemtuzumab ozogamicin in combination with vorinostat and azacitidine in older patients with relapsed or refractory acute myeloid leukemia: a phase I/II study.Haematologica. 2014 Jan;99(1):54-9. doi: 10.3324/haematol.2013.096545. Epub 2013 Oct 18. Haematologica. 2014. PMID: 24142996 Free PMC article. Clinical Trial.
-
Mcl-1 dependence predicts response to vorinostat and gemtuzumab ozogamicin in acute myeloid leukemia.Leuk Res. 2014 May;38(5):564-8. doi: 10.1016/j.leukres.2014.02.007. Epub 2014 Feb 28. Leuk Res. 2014. PMID: 24636337 Free PMC article. Clinical Trial.
-
Addition of gemtuzumab ozogamicin to induction chemotherapy in adult patients with acute myeloid leukaemia: a meta-analysis of individual patient data from randomised controlled trials.Lancet Oncol. 2014 Aug;15(9):986-96. doi: 10.1016/S1470-2045(14)70281-5. Epub 2014 Jul 6. Lancet Oncol. 2014. PMID: 25008258 Free PMC article. Review.
-
Defining the dose of gemtuzumab ozogamicin in combination with induction chemotherapy in acute myeloid leukemia: a comparison of 3 mg/m2 with 6 mg/m2 in the NCRI AML17 Trial.Haematologica. 2016 Jun;101(6):724-31. doi: 10.3324/haematol.2016.141937. Epub 2016 Feb 26. Haematologica. 2016. PMID: 26921360 Free PMC article. Clinical Trial.
-
Effect of adding gemtuzumab ozogamicin to induction chemotherapy for newly diagnosed acute myeloid leukemia: a meta-analysis of prospective randomized phase III trials.Ann Oncol. 2014 Feb;25(2):455-61. doi: 10.1093/annonc/mdt566. Ann Oncol. 2014. PMID: 24478322
Cited by
-
Histone acetyltransferases and histone deacetylases in B- and T-cell development, physiology and malignancy.Genes Cancer. 2015 May;6(5-6):184-213. doi: 10.18632/genesandcancer.65. Genes Cancer. 2015. PMID: 26124919 Free PMC article. Review.
-
Panobinostat as part of induction and maintenance for elderly patients with newly diagnosed acute myeloid leukemia: phase Ib/II panobidara study.Haematologica. 2015 Oct;100(10):1294-300. doi: 10.3324/haematol.2015.129577. Epub 2015 Jul 9. Haematologica. 2015. PMID: 26160880 Free PMC article. Clinical Trial.
-
HDAC Inhibitors in Acute Myeloid Leukemia.Cancers (Basel). 2019 Nov 14;11(11):1794. doi: 10.3390/cancers11111794. Cancers (Basel). 2019. PMID: 31739588 Free PMC article. Review.
-
The histone deacetylase inhibitor SAHA acts in synergism with fenretinide and doxorubicin to control growth of rhabdoid tumor cells.BMC Cancer. 2013 Jun 13;13:286. doi: 10.1186/1471-2407-13-286. BMC Cancer. 2013. PMID: 23764045 Free PMC article.
-
Functional Drug Screening of Small Molecule Inhibitors of Epigenetic Modifiers in Refractory AML Patients.Cancers (Basel). 2022 Aug 24;14(17):4094. doi: 10.3390/cancers14174094. Cancers (Basel). 2022. PMID: 36077629 Free PMC article.
References
-
- Howlader N, Noone AM, Krapcho M, Neyman N, Aminou R, Waldron W, et al. SEER Cancer Statistics Review, 1975–2008. National Cancer Institute; Bethesda, MD: http://seer.cancer.gov/csr/1975_2008/
-
- Döhner H, Estey EH, Amadori S, Appelbaum FR, Büchner T, Burnett AK, et al. Diagnosis and management of acute myeloid leukemia in adults: recommendations from an international expert panel, on behalf of the European LeukemiaNet. Blood. 2010;115(3):453–74. - PubMed
-
- NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines™) Acute myeloid leukemia. Version 2. 2011. Available from: www.nccn.org.
-
- Linenberger ML. CD33-directed therapy with gemtuzumab ozogamicin in acute myeloid leukemia: progress in understanding cytotoxicity and potential mechanisms of drug resistance. Leukemia. 2005;19(2):176–82. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

